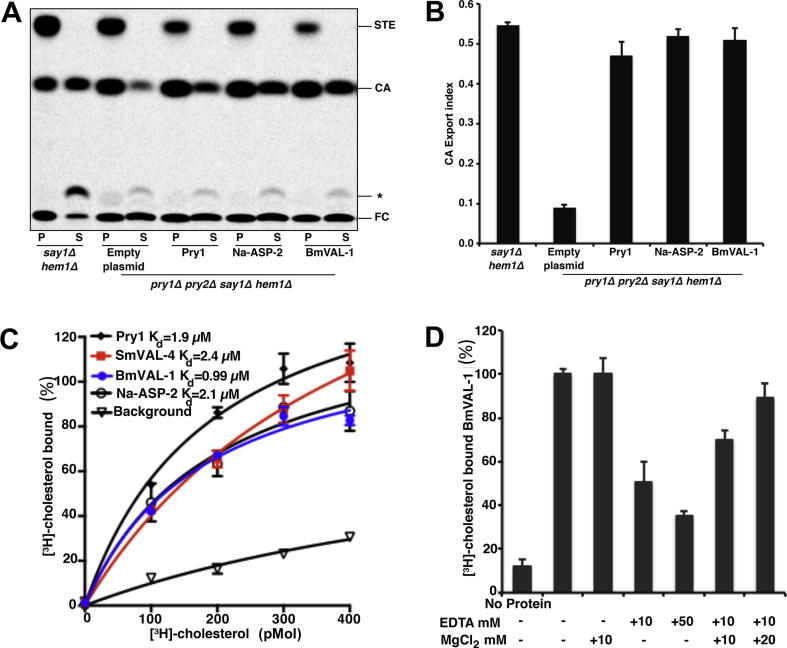
Fig. 2.
In vivo and in vitro sterol binding by Brugia malayi venom allergen-like protein 1 (BmVAL-1). (A) Expression of BmVAL-1, Necator americanus Ancylostoma secreted protein-2 (Na-ASP-2) and pathogen-related yeast protein 1 (Pry1) complement the sterol export defect of yeast cells lacking their endogenous Pry1,2. Heme-deficient cells of the indicated genotype containing either an empty plasmid or a plasmid with BmVAL-1, Na-ASP-2 or Pry1 were radiolabeled with [14C]cholesterol overnight, washed and diluted in fresh media to allow for export of cholesterol and cholesteryl acetate. Lipids were extracted from the cell pellet (P) and the culture supernatant (S), and separated by thin layer chromatography. The positions of free cholesterol (FC), cholesteryl acetate (CA) and steryl esters (STE) are indicated. The star marks the position of an unidentified cholesterol derivative. (B) Quantification of the export of cholesteryl acetate. The export index indicates the relative percentages of cholesteryl acetate that are exported by the cells (ratio between the extracellular cholesteryl acetate and the sum of intra- and extra-cellular cholesteryl acetate). Data represent mean ± S.D. of two independent experiments. (C) In vitro sterol binding by BmVAL-1 is comparable with Pry1, Schistosoma mansoni venom allergen-like protein 4 (SmVAL-4) and Na-ASP-2. (D) Addition of MgCl2 rescues the loss in sterol transport caused by the addition of EDTA.