Figure 5.
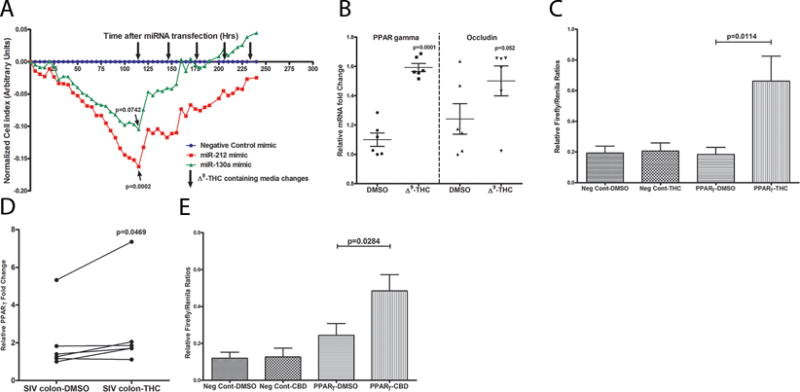
Transfection of Caco-2 cells with 30nM PPARγ targeting miR-130a (green) and OCLN targeting miR-212 (red) mimics markedly decreased TEER at 120h post transfection compared to negative control miRNA mimic (cel-miR-67-3p) transfected cells (blue) (A). MiRNA transfections were performed in quadruplicate wells and repeated three times. Note TEER restoration to control levels after two and five Δ9-THC-containing media changes (down-facing arrows) for miR-130a & miR-212, respectively. Δ9-THC significantly increased PPARγ mRNA expression (B) in Caco-2 cells by directly trans activating (C) PPARγ gene expression. To confirm that Δ9-THC stimulates PPARγ expression, Caco-2 cells were transfected with a luciferase reporter gene containing the PPARγ response element (3×PPRE-TK-Luc) and a negative control vector. Note significant increase in luciferase activity (firefly/renilla ratios) 8 h after 15 μM Δ9-THC treatment of cells transfected with 3xPPRE-TK-Luc (PPARγ) but not negative control vector (C). DMSO treatment of cells transfected with negative control vector or 3xPPRE-TK-Luc (PPARγ) vector did not increase firefly/renilla ratios. Treatment of colon tissue from chronically SIV-infected rhesus macaques with 15 μM Δ9-THC for 16 h significantly increased PPARγ mRNA expression (D). Like Δ9-THC (C), luciferase reporter assays confirmed the ability of cannabidiol, a non-psychotropic/anti-inflammatory cannabinoid to directly trans activate PPARγ expression (E). Data (B, C & E) are shown as means with error bars representing SEM.
