Figure 3.
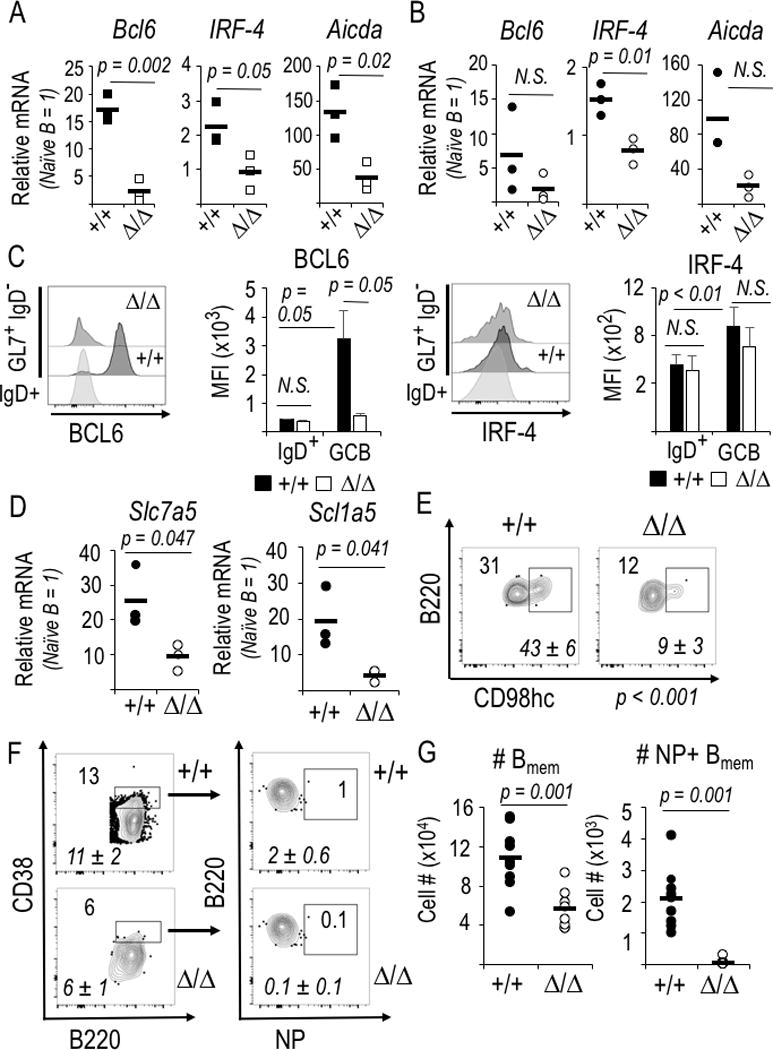
Bcl6 and Irf4 gene expression along with neutral amino acid transporters require mTORC1 in the GC-phenotype B cell. (A, B) Relative mRNA levels were quantified by qRT2-PCR using naïve, GC- and memory-phenotype B cells (huCD20-CreERT2+, either Rptor +/+ or Δ/Δ) purified from the splenocyte suspensions by flow sorting B cells according to IgD, GL7, CD38, and viability indices (FSC, SSC, and 7-AAD dye). Relative mRNA is normalized to flow-purified IgD+ GL7neg naïve B cells, whose relative mRNA level is defined as 1 for each amplification. Shown are mean (±SEM) measurements of independent samples in two biological replicate experiments, each with three each of WT (CreERT2+, Rptor+/+) and KO (CreERT2+, Rptorf/f). (A) Reduced mRNA encoding AID (Aicda) as well as BCL6 (Bcl6) and IRF4 (Irf4), transcription factors essential for GC B cell differentiation, in Raptor-depleted GL7+ B cells. (B) Reduced mRNA encoding IRF4 in Raptor-depleted memory-phenotype (IgD− GL7− CD38hi) B cells. (C) Induction of BCL6 in IgD− GL7+ B cells required mTORC1. Mice (huCD20-CreERT2+ Rptor +/+, and huCD20-CreERT2+ Rptor fl/fl)) were injected with tamoxifen and initially immunized with NP-KLH in alum as in Fig. 2A, boosted with the same amount of NP-KLH in alum one week later, harvested one week thereafter, processed as described in the Methods, followed by collection of 2 × 106 viable, dumpneg events on the flow cytometer. Shown to the left are representative flow profiles for intracellular staining of BCL6 in the GL7+ CD95+ gate or IgD+ control gate, as indicated, with B cells that were WT (+/+) or Raptor-depleted (Δ/Δ), flanked by a bar graph with the mean (±SEM) MFI for BCL6 in the replicate samples (two independent experiments, each with two mice of each genotype). To the right are the analogous data for intracellular IRF4 in splenic B cells of the same immunized mice. (D) mTORC1 dependence of large, neutral amino acid transporter gene expression in GC B cells. (E) Reduced cell surface CD98hc on Raptor-depleted GC-phenotype B cells. Representative and aggregate [italicized mean (±SEM)] flow cytometry results for IgD+ GL7neg B cells of the immunized mice. P<0.01 for the reduced CD98hc on mTORC1-depleted B cells relative to controls. (F, G) Formation of Ag-specific memory B cells requires mTORC1. (F) Representative flow phenotyping results for spleens from immunized and boosted huCD20-CreERT2 mice analyzed in Fig. 2A-E. Inset and italicized (mean ± SEM) numbers are as in Fig. 2C. (G) Absolute numbers of memory-phenotype and NP-binding memory B cells in the spleens of huCD20-CreERT2 mice, derived from phenotyping in (F).
