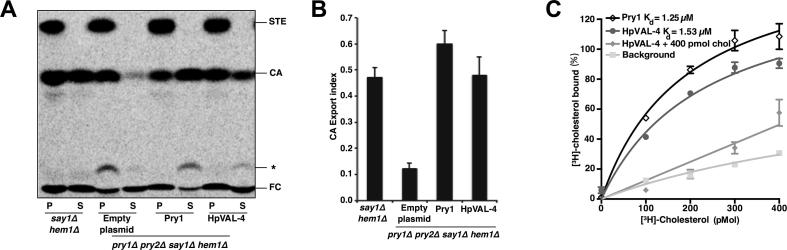
Fig. 3.
Heligmosomoides polygyrus Venom Allergen-like Protein-4 (HpVAL-4) exports and binds cholesterol in vivo and in vitro. (A) Expression of HpVAL-4 complements the sterol export defect of yeast cells lacking their endogenous cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 (CAP) proteins (Pry1 and Pry2). Heme-deficient cells of the indicated genotype containing either an empty plasmid or a plasmid with Pry1 or HpVAL-4 were radiolabeled with [14C]cholesterol overnight, washed and diluted in fresh media to allow for export of cholesterol and cholesteryl acetate. Lipids were extracted from the cell pellet (P) and the culture supernatant (S), and separated by thin layer chromatography. The positions of free cholesterol (FC), cholesteryl acetate (CA) and steryl esters (STE) are indicated. The star marks the position of an unidentified cholesterol derivative. (B) Quantification of the export of cholesteryl acetate in yeast cells lacking their endogenous CAP proteins when complemented with empty plasmid, Pry1 or HpVAL-4. The export index indicates the relative percentages of cholesteryl acetate that is exported by the cells (ratio between the extracellular cholesteryl acetate and the sum of intra- and extra-cellular cholesteryl acetate). Data represent mean ± S.D. of two independent experiments. (C) HpVAL-4 binds cholesterol in vitro. Purified HpVAL-4 protein (100 pmol) was incubated with the indicated concentration of [3H]-cholesterol in the presence (HpVAL-4 + 400 pmol cholesterol) or absence of unlabeled competitor ligand (HpVAL-4). The previously determined Pry1 activity is also shown.