Figure 2. Prolonged Confirmed PR in Patient with Advanced HBV-associated HCC.
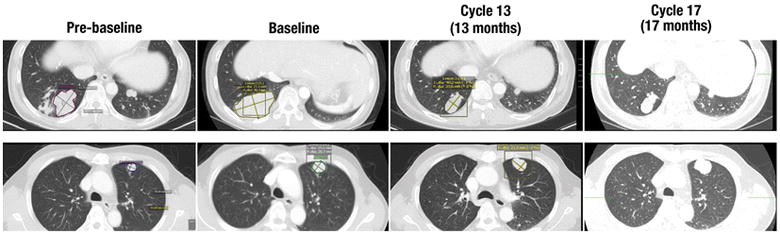
Note progressive disease (pre-baseline to baseline) in lung metastases target lesions prior to enrollment in study. MRX34 BIW 50 mg/m2 was initiated in August 2014, with stable disease for the initial 6 cycles and then prolonged confirmed PR lasting 48 weeks (34% maximum sum-of-diameters reduction for target lesions by RECIST 1.1). The patient remained in PR until cycle 17 when progression in a non-target lesion was noted. As of April 2016, the patient had received 2 additional cycles (19 total) of MRX34, with the size of both target and non-target lesions remaining stable.
