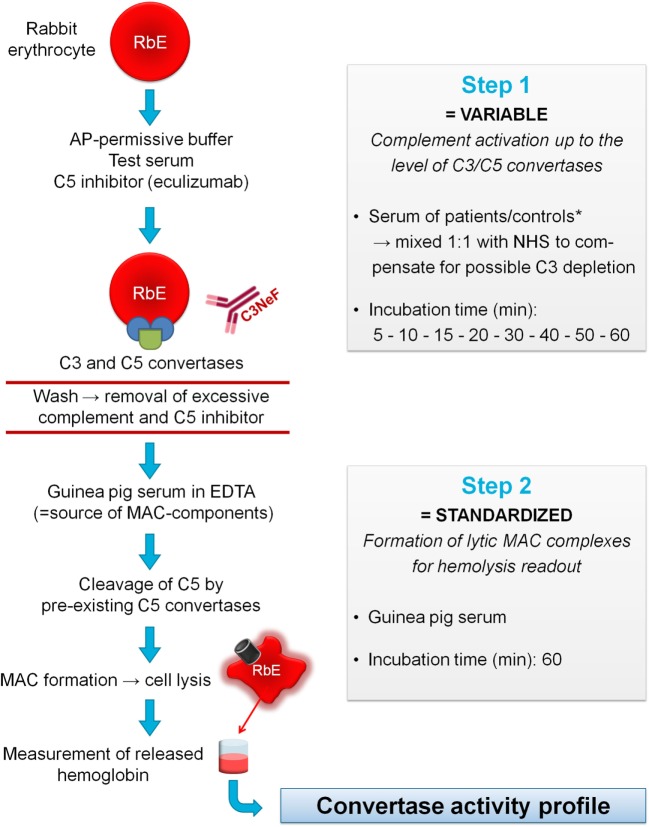
Figure 1.
Principles of the convertase activity assay for detecting convertase-stabilizing factors in serum. In step 1, rabbit erythrocytes (RbE) are incubated in an alternative pathway (AP)-permissive buffer with test serum mixed 1:1 with pooled normal human serum (NHS) to compensate for possible C3 depletion in the test sample. The C5 inhibitor eculizumab is added to halt complement activation at the level of the C3/C5 convertases. Convertase assembly and decay are followed over time using different incubation times (5–60 min). Convertase-stabilizing factors present in the sample, e.g., C3 nephritic factor (C3NeF), may interfere at this point with convertase decay. Before proceeding to step 2, erythrocytes are washed to remove remaining complement factors and C5 inhibitor. Then, convertase-bearing erythrocytes are incubated for 60 min with guinea pig serum as a source of membrane attack complex (MAC) components. The presence of ethylenediaminetetraacetic acid (EDTA) disables de novo formation of convertases from guinea pig serum and assures that only preformed convertases of the first step may initiate MAC formation and subsequent hemolysis. The released hemoglobin is quantified by spectrophotometric measurement and reflects the activity of the preformed convertases in step 1 per experimental time point. These data are used to generate convertase activity profiles over time. *If desired, immunoglobulin fractions may be added to NHS to dissect the nature of the stabilizing factor (see Materials and Methods).