Scheme 1. Synthesis of 5, 9 and 12–15 a.
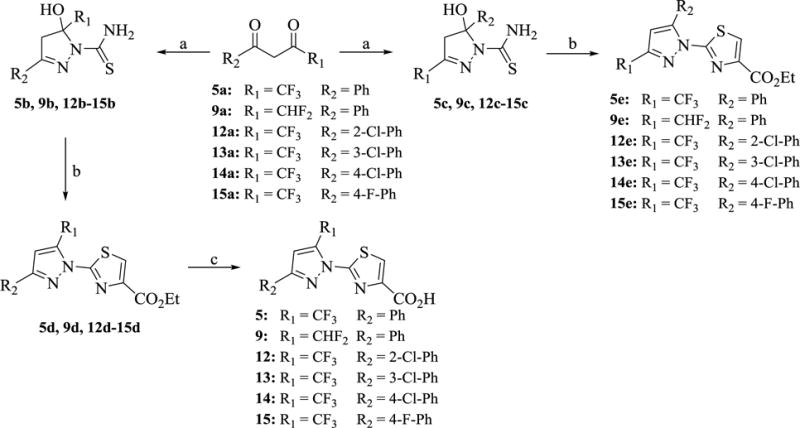
aReagents and conditions: (a) NH2CSNHNH2, EtOH, reflux, 12 h, 55–75% (b) i. BrCH2COCO2Et, EtOH, reflux, 1 h; ii. EtOH, H2SO4, reflux, 12 h, 24–35% (c) i. Reversed-phase chromatography separation of regioisomers ii. HCl, AcOH, 120 °C, 1 h.
