Scheme 2. Synthesis of precursors (I, II, and III) and analogs 7 and 10 a.
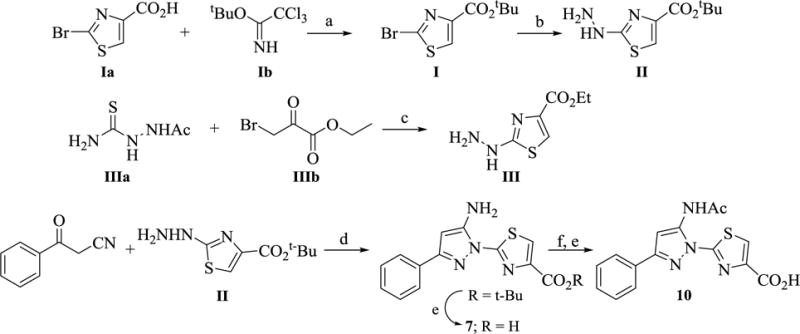
aReagents and conditions: (a) BF3·OEt2, CH2Cl2‒THF, 12 h, 88% (b) N2H4, EtOH, reflux, 2 h, 82% (c) EtOH, reflux, 5 h (d) EtOH, AcOH, reflux, 12 h, 77% (e) TFA, CH2Cl2 (f) Ac2O, Pyridine, 100 °C.
