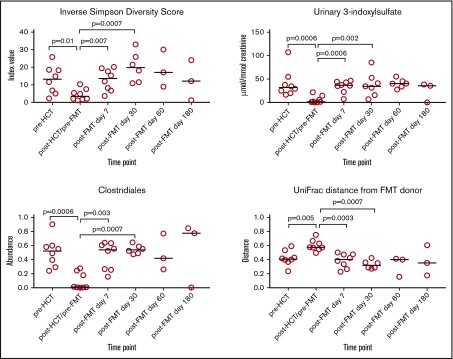
Figure 5.
Microbiome assessment under conditions that highlight the potential benefit of FMT as part of an exploratory analysis in a subset of 8 patients. Eligibility criteria for inclusion in this subset analysis received microbiome-disrupting antibiotics (ceftazidime, cefepime, piperacillin-tazobactam, meropenem, oral vancomycin, or metronidazole) before FMT, and did not receive microbiome-disrupting antibiotics in the 60 days after FMT.