Fig. 1.
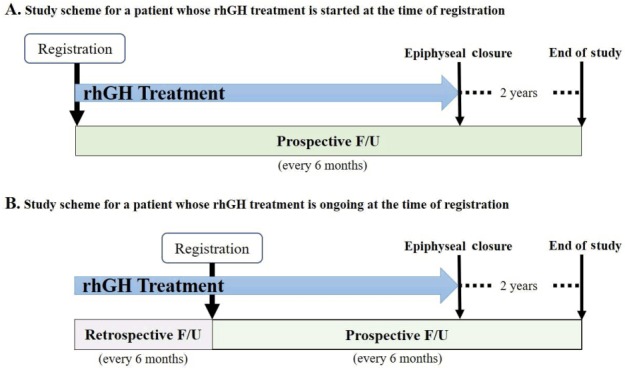
Study scheme. Study data collection is prospective in nature (A); however, if a patient is registered during the course of recombinant human growth hormone (rhGH) treatment, pretreatment (baseline) information and data obtained thereafter are collected retrospectively (B). For all patients including those who are withdrawn from the treatment before reaching adult height, the follow-up (F/U) continues until 2 years are passed after epiphyseal closure.
