Abstract
The cholesterol-lowering and anti-atherogenic effects of lemon essential oil (LEO) were investigated and compared with the effects of limonene. Owing to their volatility, both LEO and limonene were microencapsulated before preparation of the diet (20%, w/w). Hypercholesterolemia-induced rabbits were divided into 3 groups based on plasma total cholesterol (TC) levels and fed coating matrix (control group), LEO (LEO group), or limonene (Limonene group) for 8 weeks. LEO dose-dependently inhibited low-density lipoprotein oxidation in vitro. Plasma TC levels were the lowest in the LEO group (P<0.05). Erythrocytes in the LEO group had a normal disc shape, whereas the erythrocytes in the limonene and control groups were aggregated and star-shaped, respectively. The aortic intima thickness was thinnest in the LEO group followed by the control and limonene groups. Plasma TC lowering and anti-atherogenic effects of LEO were greater than limonene, suggesting that other bioactive compounds besides limonene in LEO might contribute to these effects. The bioactive compounds in LEO were limonene (67.57%), β-pinene (10.00%), and γ-terpinene (9.95%). In addition, sabinene, α-pinene, myrcene, and geranial were also present but the amount was in the range of 1~2%. Several bioactive compounds were also detected. In conclusion, LEO had beneficial effects on hypercholesterolemia due to its antioxidative and cholesterol lowering effects.
Keywords: lemon essential oil, limonene, antioxidant, lipid lowering, hypercholesterolemia
INTRODUCTION
Essential oils, in aromatic plants, have pharmacological activities, including antibacterial, antimicrobial, antifungal, and antiparasite effects, which might be from secondary metabolites extracted along with the oil during processing (1). Being low-molecular-weight compounds, essential oils can easily penetrate the cell membrane and therefore be involved in biochemical reactions in the body (2). Studies on the health benefits of essential oils have focused mainly on their antioxidative (3), anti-inflammatory (4), lipid-lowering (5,6), antidiabetic (7), and hepatic protective effects (8,9). Although almost 3,000 varieties of essential oils are available, only 10% are used as pharmaceutical materials, food additives, or ingredients for cosmetics and fragrances (10). Among essential oils, those from citrus fruits are widely used in industry, because of availability of leftover fruit skin. Lemon (Citrus limon L. Burm), orange (Citrus sinensis L. Osbeck), bitter orange (Citrus aurantium L.), bergamot (Citrus bergamia), mandarin (Citrus deliciosa Tenore), and grape fruit (Citrus paradise Macfayden) are the major fruits used. Lemon essential oil (LEO), in particular, has shown antifungal (11) and antioxidative activities (12), as well as improved hippocampus function (13). The bioactive compounds in LEO are limonene, β-pinene, and γ-terpinene. In addition, minor components such as α-pinene, myrcene, and geraniol are present (14). Limonene, a major compound in LEO, showed lipid-lowering effects via upregulation of peroxisome proliferator-activated receptor alpha (PPARα) and liver X receptor beta (LXRβ) (15). γ-Terpinene also had a lipid-lowering effect (16).
Hypercholesterolemia plays a pivotal role in the development of atherosclerosis, which is a leading cause of death. Consequently, extensive studies have been conducted on the development of therapeutic drugs to prevent atherosclerosis; however, drug side effects are a big concern (17). Functional foods or alternative medicines, such as essential oils, have drawn attention as alternatives to reduce the problems from drug use. Although the lipid-lowering effects of bioactive compounds in essential oils, particularly limonene, have been extensively examined, the hypolipidemic and anti-atherogenic effects of LEO have not yet been studied. The industrial use of LEO is expected to be high because the cost-benefit is higher than that of other essential oils. Moreover, various bioactive compounds, including limonene, are present in LEO.
In this study, the lipid-lowering and anti-atherogenic effects of LEO were investigated in hypercholesterolemia-induced rabbits and compared with the effects of limonene. The rabbit is an animal model used in atherosclerosis research because the animal is prone to developing hypercholesterolemia, which is a critical factor in the pathogenesis of atherosclerosis. In this study, LEO and limonene were microencapsulated for diet preparations because they are highly volatile.
MATERIALS AND METHODS
Microencapsulation of LEO and limonene
LEO was purchased from Perfect Potion (Brisbane, Australia), whereas limonene of 97% optical purity was purchased from Sigma-Aldrich Co. (183164; St. Louis, MO, USA). According to the manufacturer’s information, lemon fruit skin had been cold pressed to extract the oil. Because of their high volatility, both LEO and limonene were microencapsulated before use (Sejeon Co., Chungbuk, Korea). For the microencapsulation, 88% of modified starch and 12% of maltodextrin were used as coating materials (18). The amount of essential oil in the microencapsulated powder was 20% (w/w).
Gas chromatography (GC) of LEO
The bioactive compounds in LEO were analyzed using a GC system (HP-6890; Hewlett-Packard Co., Palo Alto, CA, USA) equipped with a flame ionization detector. The oven temperature was set to change over time as follows: initial temperature of 80°C for 10 min, then 5°C/min increments up to 150°C, and finally 10°C/min increments up to 250°C.
Determination of low-density lipoprotein (LDL) susceptibility to oxidation
LDL (L-5402; Sigma-Aldrich Co.) was dialyzed for 24 h at 4°C in phosphate-buffered saline (PBS), then filtered through a 0.45-m syringe filter, and finally quantified using a protein assay kit (#5000002EDU; Bio-Rad Laboratories Inc., Hercules, CA, USA). The LDL oxidation reaction solution consisted of 0.1 mg/mL protein, alcohol, and Cu2+ (final concentration of 5 μM), into which the LEO or vitamin E dissolved in alcohol was added. The reaction solution was oxidized for 4 h at 40°C, and then mixed with 1 mL of thiobarbituric acid reactive substances (TBARS) solution [0.4% 2-thiobarbituric acid (TBA), 15% trichloroacetic acid, and 2.5% HCl] and boiled for 20 min at 95~100°C. Thereafter, the hot mixture was immediately cooled on ice and then centrifuged for 10 min at 3,012.4 g. The absorbance of the supernatant fraction was measured at 532 nm. The TBARS value was expressed as the malondialdehyde (MDA; nmol/mL) amount. The calculated inhibition effect (%) was based on the percentage of LDL oxidation.
Animals and experimental diets
Twenty-one specific pathogen-free rabbits (1,860±114.3 g, male) purchased from Orient Co., Ltd. (Seoul, Korea) were acclimated individually for 2 weeks in stainless steel cages at a controlled temperature of 23±2°C, relative humidity of 55±5%, and 12-h light/dark cycle. After acclimation, the rabbits were fed a high-cholesterol diet (HCD) for 4 weeks to induce hypercholesterolemia. The HCD was prepared by adding 0.5% (w/w) cholesterol and 10% (w/w) coconut oil to chow diet (Purina Rat Chow, #5001; Ralston Purina, St. Louis, MO, USA). After 4 weeks, the cholesterol level was determined from blood drawn from the ear vein. Hypercholesterolemia was confirmed as a cholesterol level >1,000 mg/mL (19). The hypercholesterolemic rabbits were assigned to three groups (n=7 per group) based on plasma cholesterol concentration. The microencapsulation coating material was used as the control group to avoid bias effects from microencapsulation. In addition, the facility for rabbit experiments, using automatic-feeding and -watering cages, was limited to a certain number of rabbits since they had to be house individually. The experimental groups were: rabbits fed a HCD containing coating material (Control group), microencapsulated LEO (LEO group), or microencapsulated limonene (Limonene group), respectively. The amount of coating material and microencapsulated power added to the HCD was 2.5% (w/w). Rabbits demonstrated toxicity at 500 mg/kg body weight of oral administration (7,20). Therefore, in this study, we provided 250 mg/kg body weight of LEO or limonene to the rabbits. The diet was prepared every week and kept at −18°C. The diet provided to the animals for 8 weeks was restricted, whereas water was provided ad libitum. After the 8 weeks of feeding, the rabbits were fasted for 12 h and anesthetized with a tiletamine-zolazepam solution (15 mg/kg; Zoletil 50; Bayer Korea, Seoul, Korea). Blood obtained from the inferior vena cava was separated into heparin tubes for lipid concentration analysis or into ethylenediaminetetraacetic acid (EDTA) tubes for erythrocyte analysis. After perfusing with ice-cold PBS (pH 7.4), the liver, heart, and aorta were excised, rinsed, and weighed. The plasma and organs were stored at −80°C for further analysis. The animal study was approved by the Pusan National University-Institutional Animal Care and Use Committee (PNU-2013-0375).
Determination of plasma lipid concentrations
Each plasma sample was separated by centrifugation for 10 min at 3,012.4 g at 4°C. Total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) levels were measured using commercial kits (AM 202-K, AM 157S-K, and AM 203-K, respectively; Asan Pharmaceutical Co., Seoul, Korea). LDL cholesterol (LDL-C) levels were calculated using the Friedewald equation (21).
Analysis of lipid peroxidation in the plasma
Plasma lipid peroxidation was measured and expressed as TBARS values in MDA (22). Briefly, plasma was mixed with TBARS solution (0.67% TBA and 0.05 N HCl) and incubated at 95°C for 30 min. The reaction was terminated by the addition of n-butanol/methanol solution. The reaction mixture was centrifuged at 18,627 g for 10 min, and the UV absorbance of the upper layer was measured at 540 nm using a spectrophotometer. MDA was used as the standard.
Determination of antioxidants in the liver
Hepatic catalase (23) and glutathione peroxidase (GSH-Px) (24) activities were determined according to previously reported methods. Briefly, the substrates used for the determination of catalase and GSH-Px activities were hydrogen peroxide and nicotinamide adenine dinucleotide phosphate, respectively. Bovine serum albumin was used for protein quantification.
Analysis of erythrocyte morphology
Blood samples drawn into EDTA tubes were diluted in PBS, and the erythrocytes were observed under an automated microscope (100×) equipped with an image analyzer (Olympus CH30; Olympus, Tokyo, Japan) (25).
Fatty streak formation in the aorta
A 3-cm length of the descending aorta was cut from the heart. The aorta was opened, fixed in 4% paraformaldehyde overnight at 4°C, and then rinsed in 30% sucrose solution and PBS (10 M, pH 7.2). The tissue was embedded in paraffin, which was then sectioned into 4-μm thickness using a cryostat (Leica CM 3050; Leica Biosystems Nussloch GmbH, Nußloch, Germany) and mounted on a coated slide glass. To evaluate the changes in the intima thickness, the sequential sections were stained with hematoxylin and eosin, and the stained slides were monitored under a camera-mounted microscope (Olympus CH30; Olympus).
Statistical analysis
Values were expressed as the mean±standard deviation (SD). Statistical analysis was performed using the SAS program (SAS Institute Inc., Cary, NC, USA). One-way analysis of variance was carried out, followed by Duncan’s multiple range test. Significance was considered at P<0.05.
RESULTS
Bioactive compounds in LEO
The bioactive compounds in LEO, as analyzed by GC, are shown in Table 1. The amount of limonene, β-pinene, and γ-terpinene in LEO was 66.57%, 10.00%, and 9.95%, respectively, indicating that limonene is the major bioactive compound. Besides these, sabinene, α-pinene, myrcene, and geraniol were present in a range of 1~2%. Seven other bioactive compounds were also detected in small quantities.
Table 1.
Bioactive compounds in the lemon essential oil used in this study
| Compound | Relative area (%) |
|---|---|
| Limonene | 66.57 |
| β-Pinene | 10.00 |
| γ-Terpinene | 9.95 |
| Sabinene | 1.60 |
| α-Pinene | 1.95 |
| Myrcene | 1.59 |
| geraniol | 1.17 |
| Neral | 0.87 |
| β-Bisabolene | 0.59 |
| Neryl acetate | 0.53 |
| trans-α-Bergamotene | 0.43 |
| Geranyl acetate | 0.35 |
| Terpinolene | 0.29 |
| α-Terpineol | 0.12 |
Inhibition of LDL susceptibility to oxidation
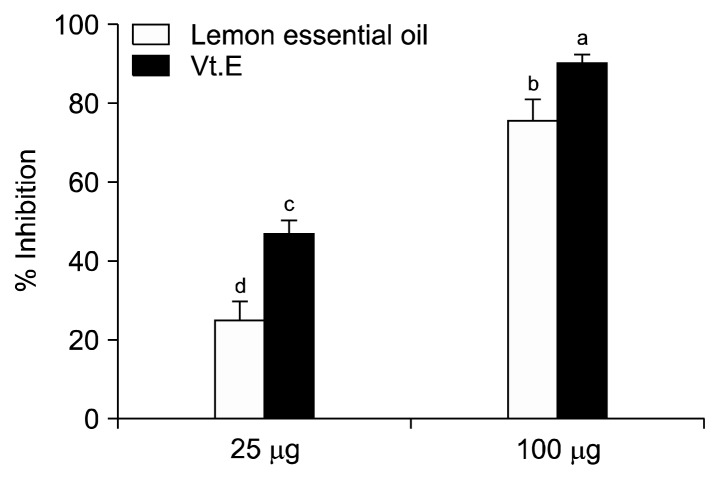
At the LEO concentrations of 25 and 100 μg, LDL susceptibility to oxidation was reduced by 24.68% and 75.64%, respectively (Fig. 1, P<0.05). At the same concentrations, vitamin E inhibited LDL oxidation by 47.11% and 90.22%, respectively.
Fig. 1.
Inhibition effect of lemon essential oil and vitamin E (Vt.E) on low-density lipoprotein oxidation. Data with different letters (a–d) are significantly different according to one-way ANOVA, followed by Duncan’s multiple range test at P<0.05.
Changes in body and organ weights
The initial body weight of the rabbits was about 1,800 g, which increased to 2,800±100 g after 12 weeks of the experiment, including 4 weeks for the hypercholesterolemia-induction period. Body weight gain and liver and aorta weight of rabbits were not different among experimental groups because rabbits were on the restricted diet (data not shown).
Changes in plasma lipid concentration and lipid peroxidation
Plasma TC concentration of rabbits reached to 1,285 mg/dL after 4 weeks of HCD feeding, indicating that hypercholesterolemia had been successfully induced. After 8 weeks of experimental diet feeding, the increased plasma TC concentration was significantly reduced by 18.2% in the LEO group relative to that in the control group (Table 2, P<0.05). Although the TC concentration in the limonene group had decreased by 12.8% relative to the control group, the changes were not significant. The concentrations of LDL-C and HDL-C among the three experimental groups were not different, although slight decreases were found in the LEO and limonene groups. The plasma TG concentration decreased by 27.3% in the LEO group and 25.2% in the limonene group relative to that of the control group, but the differences were not significant.
Table 2.
Effect of lemon essential oil on lipid concentration and TBARS concentration in hypercholesterolemia-induced rabbits during 8 weeks
| Group | TG (mg/dL) | TC (mg/dL) | LDL-C (mg/dL) | HDL-C (mg/dL) | TBARS (nmol MDA/mL) |
|---|---|---|---|---|---|
| Control | 385±102NS | 1,395±134a | 1,181±169NS | 25.0±1.7ab | 15.2±5.1ab |
| LEO | 280±154 | 1,142±293b | 1,067±289 | 27.7±5.1a | 11.9±2.3b |
| Limonene | 288±99 | 1,217±134a | 1,129±206 | 22.2±1.9b | 17.8±7.5a |
Control, coating material group fed a HCD (high-cholesterol diet) containing 2.5% coating materials for 8 weeks; LEO, lemon essential oil group fed a HCD containing 2.5% lemon powder; Limonene, limonene group fed a HCD containing 2.5% limonene powder. TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TBARS, thiobarbituric acid reactive substances; MDA, malondialdehyde.
Values are not significantly different among the experimental groups.
Data are the mean±SD (n=7).
Data with different letters (a,b) in the same column are significantly different according to one-way ANOVA, followed by Duncan’s multiple range test at P<0.05.
As shown in Table 2, the plasma TBARS level of the limonene group was significantly higher than the LEO group (P<0.05), indicating that limonene showed pro-oxidant activity. On the contrary, the TBARS level of the LEO group was lower than the control group although significant differences were not found.
Antioxidant levels in the liver
As shown in Table 3, the hepatic antioxidants levels such as catalase and GSH-Px were higher in the LEO group, followed by the limonene and control groups. The antioxidant activities among samples were not significant.
Table 3.
Antioxidants levels in the liver
| Group1) | Catalase (mUnits/mg protein) | GSH-Px (mUnits/mg protein) |
|---|---|---|
| Control | 0.251±0.03NS | 17.859±5.11NS |
| LEO | 0.263±0.05 | 25.805±0.48 |
| Limonene | 0.203±0.08 | 19.111±1.92 |
See the footnote of Table 2 for the experimental groups.
GSH-Px, glutathione peroxidase.
Data are the mean±SD (n=7).
Values are not significantly different among the experimental groups.
Morphological changes in erythrocyte and aortic intima due to hypercholesterolemia
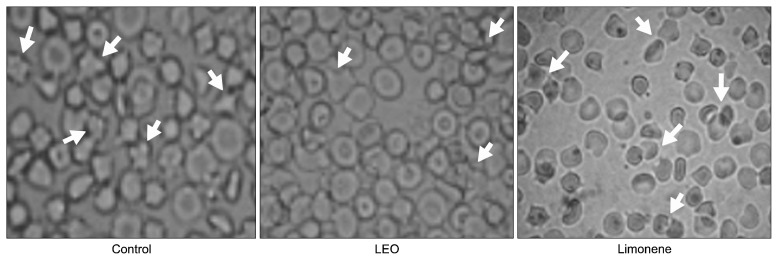
As shown in Fig. 2, erythrocytes in the control group showed aggregation and were star-like shapes, whereas those in the LEO group were of a normal disc shape. In the limonene group, star-like shapes of erythrocytes were also observed, but it was not as severe as those shown in the control group. According to these results, LEO showed protective effects against red blood cell damaging by hypercholesterolemia, which was found to be greater than that of limonene.
Fig. 2.
Morphological changes in erythrocytes of hypercholesterolemia-induced rabbits fed lemon essential oil for 8 weeks (×100). See the footnote of Table 2 for the experimental groups. Arrows indicate the changed or aggregated erythrocytes.
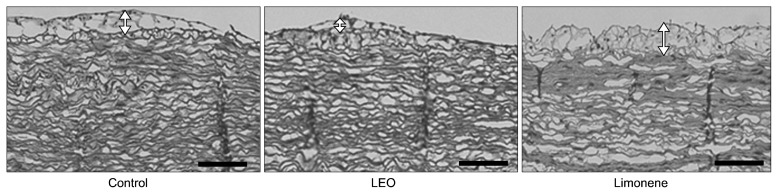
As shown in Fig. 3, the aortic intima width was the thinnest in the LEO group, followed by the control group and limonene group. These results are in line with the lipid peroxidation results and morphological changes seen in the red blood cells.
Fig. 3.
Morphological changes of the aortic intima thickness in hypercholesterolemia-induced rabbits fed lemon essential oil for 8 weeks (×100). The scale bar represents 100 μm. See the footnote of Table 2 for the experimental groups. The arrows indicate the intima thickness of each group.
DISCUSSION
Citrus fruit essential oils contain numerous bioactive compounds, such as limonene, β-pinene, and γ-terpinene, which have antioxidative (2), antibacterial (26), and lipid-lowering (27) activities. In this study, we investigated the lipid-lowering and anti-atherogenic effects of LEO in comparison with limonene, a major active compound in LEO. LDL susceptibility to oxidation is noticed in the development of atherosclerosis. Macrophages in the aorta have receptors for oxidized LDL, and agents that can inhibit LDL oxidation in the plasma have been considered in cardiovascular disease research (28). In this study, a dose-dependent inhibition of LDL susceptibility to oxidation was caused by LEO, the effects of which were comparable to those of vitamin E. Approximately 80% of the antioxidative activity of vitamin E was found at a concentration of 100 μg. Our finding is in line with previous studies that reported the antioxidative activity of citrus essential oil in both Cu2+-induced and 2,2′-azobis (2-aminopropane) hydrochloride-induced oxidation of human LDL in vitro (29). This in vitro antioxidative activity of LEO was confirmed in our in vivo study performed with rabbits, where plasma lipid peroxidation was significantly lower in the LEO group than in the limonene group. These results indicate that synergic effects from bioactive compounds, including limonene and γ-terpinene, rather than a single compound in LEO, might be responsible for the inhibitory effects. The antioxidative activity of γ-terpinene was 3-fold higher than that of limonene in the methyl linoleate oxidation system (30). Fourteen different bioactive compounds were detected from LEO used in this study. It is well-known that lipid peroxidation could increase oxidative stress that causes degenerative diseases, such as aging, atherosclerosis, and cancer through damage of zymoproteins, RNAs, and DNAs (31).
Plasma lipid levels, particularly cholesterol and LDL-C levels are positively associated with atherosclerosis development due to hypercholesterolemia. In this study, LEO decreased plasma TC levels, which was significantly higher than the effects from limonene or the coating material. In addition, the HDL-C level was significantly increased in the limonene group. Moreover, LDL-C levels in the LEO and limonene groups were reduced although no significances were found. Limonene had cholesterol-lowering effects in hypercholesterolemia-induced animals (15,32) via downregulation of PPARα and LXRβ (15). Moreover, γ-terpinene in LEO, has exhibited cholesterol-lowering effects in animals (16). Hypercholesterolemia causes morphological changes in erythrocytes (33) and promotes aortic intima proliferation, which are the major causes of cardiovascular disorders and degenerative diseases (34). In the present study, erythrocytes in the control group were deformed into star-like shapes, whereas most of the erythrocytes in the LEO group were found to be normal disc-shape. However, erythrocytes in the limonene group were aggregated and deformed into star-like shape. Antioxidants are important to help prevent oxidation in red blood cells (35). In LEO, α- and γ-terpinenes and terpinolene were identified to have antioxidant activity (2). The cholesterol-lowering effects of LEO might affect the cholesterol level in the membrane of red blood cells. The cholesterol level of red blood cells is correlated to that of non-esterified cholesterol in the blood. Non-esterified cholesterol is regulated by lecithin cholesterol acyl transferase (LCAT). Hypercholesterolemia impairs the LCAT activity, which stimulates cholesterol accumulation in the inner leaf of the red blood cell membrane. Lipid peroxidation of the membrane might therefore deform the erythrocytes (35). In this study, the TC and LDL-C concentrations in the LEO group were significantly lower than the levels in the control or limonene groups. In addition, TBARS level in the LEO group was lower than the control group. Taken together, it is possible that LEO prevented cholesterol accumulation in the red blood cells and mediated a reduction in plasma cholesterol. The bioactive compounds in LEO had antioxidative activity against lipid peroxidation on the membrane of red blood cells.
Hypercholesterolemia leads to atherosclerosis through proliferation of the aortic intima. In this study, the aortic intima thickness was thinnest in the LEO group followed by the control group and limonene group. These aortic thickness findings are in agreement with our plasma cholesterol levels and degree of peroxidation results. Lipid peroxidation in the limonene group was found to be the most severe among the experimental groups. The intimal thickening caused by foam cell formation and vascular smooth muscle proliferation is affected by inflammation and the immune response at the intima as well as the oxidized LDL (17). The antioxidative activities of bioactive compounds in LEO such as γ-terpinene, terpinolene, and α-terpineol are responsible for the anti-atherogenic effect of LEO.
In this study, we found antioxidative and cholesterol-lowering activities of LEO in hypercholesterolemia-induced rabbits, evidenced by its inhibition effects on LDL oxidation and lipid peroxidation and preventive effects against erythrocyte deformation and aortic intimal proliferation. These effects were greater than those of limonene, a major bioactive compound in LEO, suggesting that the synergistic effects of bioactive compounds in LEO were stronger than the effects of limonene alone. Besides limonene, 13 other bioactive compounds (including sabinene, β-pinene, γ-terpinene, α-pinene, myrcene, and geraniol) were detected. In particular, the antioxidative effect of γ-terpinene has been reported to be 3-fold higher than that of limonene. Unfortunately, we were unable to include many experimental groups in this study because of the limited research facilities available. In order to avoid bias effects from microencapsulation, which was an essential procedure for the preparation of highly volatile oils prior to addition in the experimental diet, we included the coating material fed-rabbits as the control group. Future research studies are needed to elucidate the mechanisms through which LEO prevents atherosclerosis as well as to determine its effect on inflammation and immune responses.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Solórzano-Santos F, Miranda-Novales MG. Essential oils from aromatic herbs as antimicrobial agents. Curr Opin Biotechnol. 2012;23:136–141. doi: 10.1016/j.copbio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Misharina TA, Samusenko AL. Antioxidant properties of essential oils from lemon, grapefruit, coriander, clove, and their mixtures. Prikl Biokhim Mikrobiol. 2008;44:438–442. [PubMed] [Google Scholar]
- 3.Sharififar F, Derakhshanfar A, Dehghan-Nudeh G, Abbasi N, Abbasi R, Gharaei RR, Koohpayeh A, Daneshpajouh M. In vivo antioxidant activity of Zataria multiflora Boiss essential oil. Pak J Pharm Sci. 2011;24:221–225. [PubMed] [Google Scholar]
- 4.Monteiro MVB, de Melo Leite AK, Bertini LM, de Morais SM, Nunes-Pinheiro DCS. Topical anti-inflammatory, gastroprotective and antioxidant effects of the essential oil of Lippia sidoides Cham. leaves. J Ethnopharmacol. 2007;111:378–382. doi: 10.1016/j.jep.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Kim HJ, Kang HJ, Seo JY, Lee CH, Kim YS, Kim JS. Antiobesity effect of oil extract of ginseng. J Med Food. 2011;14:573–583. doi: 10.1089/jmf.2010.1313. [DOI] [PubMed] [Google Scholar]
- 6.Abdollahi M, Salehnia A, Mortazavi SH, Ebrahimi M, Shafiee A, Fouladian F, Keshavarz K, Sorouri S, Khorasani R, Kazemi A. Antioxidant, antidiabetic, antihyperlipidemic, reproduction stimulatory properties and safety of essential oil of Satureja Khuzestanica in rat in vivo: a toxicopharmacological study. Med Sci Monit. 2003;9:BR331–BR335. [PubMed] [Google Scholar]
- 7.Chung MJ, Cho SY, Bhuiyan MJ, Kim KH, Lee SJ. Antidiabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br J Nutr. 2010;104:180–188. doi: 10.1017/S0007114510001765. [DOI] [PubMed] [Google Scholar]
- 8.Bak MJ, Jun M, Jeong WS. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. Int J Mol Sci. 2012;13:2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tranchida PQ, Bonaccorsi I, Dugo P, Mondello L, Dugo G. Analysis of Citrus essential oils: state of the art and future perspectives. A review. Flavour Fragrance J. 2012;27:98–123. doi: 10.1002/ffj.2089. [DOI] [Google Scholar]
- 10.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 11.Guerra FQS, Mendes JM, de Oliveira WA, de Souza FS, Trajanov VN, Coutinho HDM, de Oliveira Lima E. Antibacterial activity of the essential oil of Citrus limon against multidrug resistant Acinetobacter strains. Rev Bras Farm. 2013;94:142–147. [Google Scholar]
- 12.Bertuzzi G, Tirillini B, Angelini P, Venanzoni R. Antioxidative action of Citrus limonum essential oil on skin. Eur J Med Plants. 2013;3:1–9. doi: 10.9734/EJMP/2013/1987. [DOI] [Google Scholar]
- 13.Lopes Campêlo LM, Moura Gonçalves FC, Feitosa CM, de Freitas RM. Antioxidant activity of Citrus limon essential oil in mouse hippocampus. Pharm Biol. 2011;49:709–715. doi: 10.3109/13880209.2010.541924. [DOI] [PubMed] [Google Scholar]
- 14.Fisher K, Phillips C. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Technol. 2008;19:156–164. doi: 10.1016/j.tifs.2007.11.006. [DOI] [Google Scholar]
- 15.Jing L, Zhang Y, Fan S, Gu M, Guan Y, Lu X, Huang C, Zhou Z. Preventive and ameliorating effects of citrus D-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur J Pharmacol. 2013;715:46–55. doi: 10.1016/j.ejphar.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y, Inaba N, Kuwahara S, Kuki W. Effects of γ-terpinene on lipid concentrations in serum using Triton WR1339-treated rats. Biosci Biotechnol Biochem. 2003;67:2448–2450. doi: 10.1271/bbb.67.2448. [DOI] [PubMed] [Google Scholar]
- 17.Salvamani S, Gunasekaran B, Shukor MY, Zuki Abu Bakarb M, Ahmad SA. Phytochemical investigation, hypocholesterolemic and anti-atherosclerotic effects of Amaranthus viridis leaf extract in hypercholesterolemia-induced rabbits. RSC Adv. 2016;6:32685–32696. doi: 10.1039/C6RA04827G. [DOI] [Google Scholar]
- 18.Baranauskiene R, Bylaite E, Zukauskaite J, Venskutonis RP. Flavor retention of peppermint (Mentha piperita L.) essential oil spray-dried in modified starches during encapsulation and storage. J Agric Food Chem. 2007;55:3027–3036. doi: 10.1021/jf062508c. [DOI] [PubMed] [Google Scholar]
- 19.Kusunoki J, Hansoty DK, Aragane K, Fallon JT, Badimon JJ, Fisher EA. Acyl-CoA:cholesterol acyltransferase inhibition reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;103:2604–2609. doi: 10.1161/01.CIR.103.21.2604. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Environmental Assessment, U.S. Environmental Protection Agency. [accessed Dec 1993];Integrated Risk Information System (IRIS) Chemical Assessment Summary. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0682_summary.pdf.
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Ferré N, Camps J, Paul A, Cabré M, Calleja L, Osada J, Joven J. Effects of high-fat, low-cholesterol diets on hepatic lipid peroxidation and antioxidants in apolipoprotein E-deficient mice. Mol Cell Biochem. 2001;218:165–169. doi: 10.1023/A:1007296919243. [DOI] [PubMed] [Google Scholar]
- 23.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;7:952–958. doi: 10.1016/0006-291X(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 25.Horiuchi K, Ohata J, Hirano Y, Asakura T. Morphologic studies of sickle erythrocytes by image analysis. J Lab Clin Med. 1990;115:613–620. [PubMed] [Google Scholar]
- 26.Soković M, Glamočlija J, Marin PD, Brkić D, van Griensven LJ. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miceli N, Mondello MR, Monforte MT, Sdrafkakis V, Dugo P, Crupi ML, Taviano MF, de Pasquale R, Trovato A. Hypolipidemic effects of Citrus bergamia Risso et Poiteau juice in rats fed a hypercholesterolemic diet. J Agric Food Chem. 2007;55:10671–10677. doi: 10.1021/jf071772i. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg D, Witztum JL. Lipoproteins and atherogenesis: current concepts. JAMA. 1990;264:3047–3052. doi: 10.1001/jama.1990.03450230083034. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi Y, Inaba N, Kuwahara S, Kuki W. Antioxidative effect of citrus essential oil components on human low-density lipoprotein in vitro. Biosci Biotechnol Biochem. 2003;67:195–197. doi: 10.1271/bbb.67.195. [DOI] [PubMed] [Google Scholar]
- 30.Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69:167–174. doi: 10.1016/S0308-8146(99)00247-2. [DOI] [Google Scholar]
- 31.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- 32.Santiago JVA, Jayachitra J, Shenbagam M, Nalini N. d-Limonene attenuates blood pressure and improves the lipid and antioxidant status in high fat diet and L-NAME treated rats. J Pharm Sci Res. 2010;2:752–758. [Google Scholar]
- 33.Kanakaraj P, Singh M. Influence of hypercholesterolemia on morphological and rheological characteristics of erythrocytes. Atherosclerosis. 1989;76:209–218. doi: 10.1016/0021-9150(89)90105-6. [DOI] [PubMed] [Google Scholar]
- 34.Schmid-Schönbein H. Microrheology of erythrocytes, blood viscosity, and the distribution of blood flow in the microcirculation. Int Rev Physiol. 1975;9:1–62. [PubMed] [Google Scholar]
- 35.Vatsala TM, Singh M. Changes in shape of erythrocytes in rabbits on atherogenic diet and onion extracts. Atherosclerosis. 1980;36:39–45. doi: 10.1016/0021-9150(80)90196-3. [DOI] [PubMed] [Google Scholar]