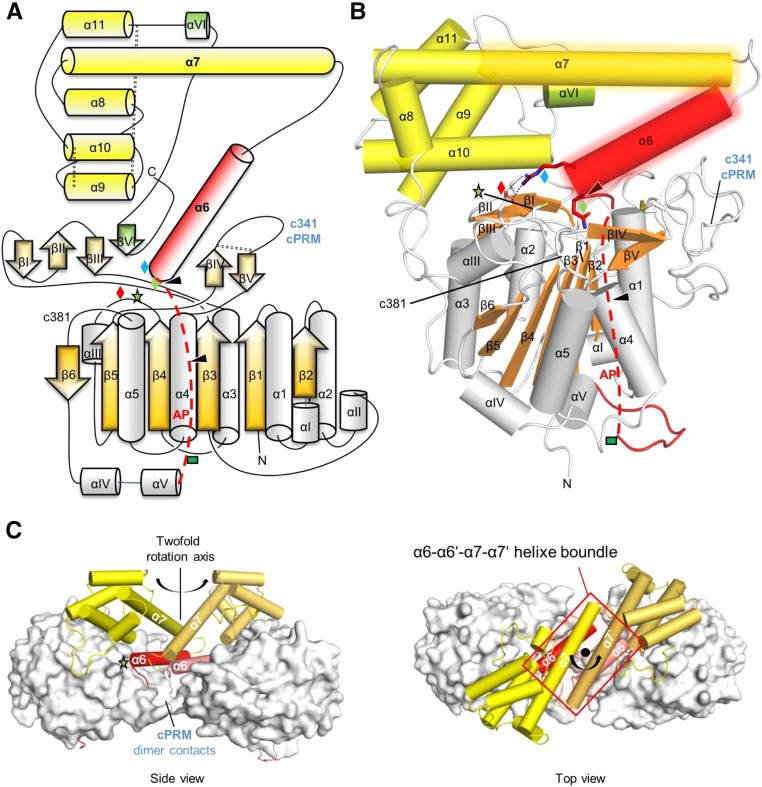
Figure 1.
Topology and Domain Organization of Two-Chain AtLEGγ.
(A) Topology diagram of AtLEGγ. The catalytic domain is colored in gray and orange, the activation peptide (AP) including the α6-helix in red, the LSAM in yellow, and the putative VSS in green. The red dashed line indicates part of the AP, which would be intact in the precursor form. The blue, red, and green diamonds and the green star represent Arg-355, Glu-220, Gln-354, and the catalytic cysteine (Cys-219), respectively. The green rectangle represents an N-glycosylation site. Autocleavage sites are indicated as black triangles and the three disulphide bridges by black dashed lines.
(B) The X-ray structure of AtLEGγ monomer molecule in cartoon representation with identical color code as in (A). Dimer interface regions are shaded areas around the α6- and α7-helices.
(C) Dimeric structure of two-chain AtLEGγ. Catalytic domains are displayed as surface, the AP, including the α6-helix, and the LSAM as red and yellow cartoons, respectively. The cPRM contacts within the dimer are highlighted. Left: Front view, perpendicular to the 2-fold axis. Down: Top view along the 2-fold axis.