Figure 4.
The α6-Helix Is Highly pH-Dependently Regulated.
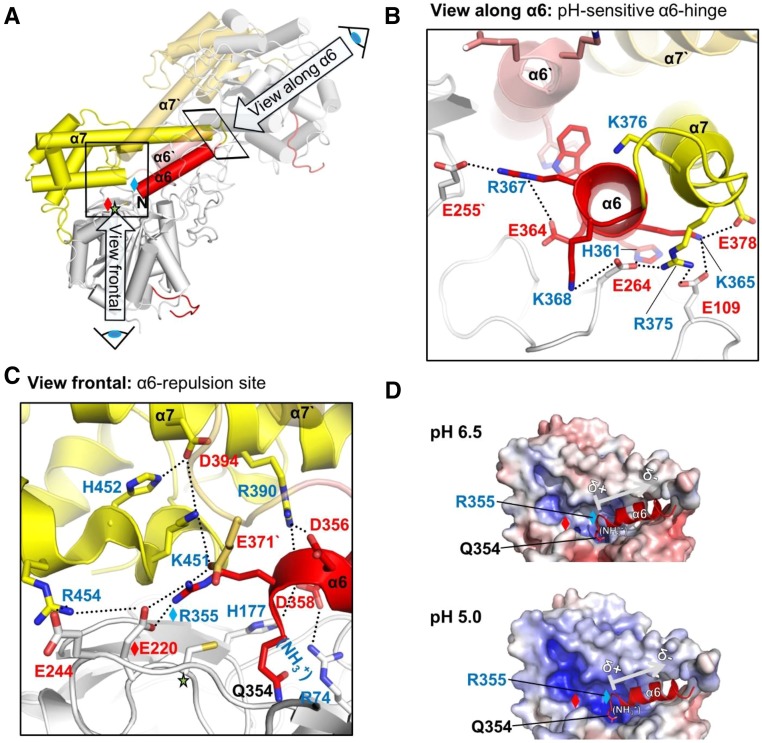
(A) Overall view of the two-chain AtLEGγ dimer, indicating the two pH-sensitive areas of the four-helix bundle α6, α7, α6′, α7′. The catalytic domain, LSAM, and α6-helix are colored in gray, yellow, and red, respectively.
(B) Close-up view onto the pH-sensitive α6 hinge region, highlighting the intra- and intermolecular electrostatic clamping of the α6-helix, which is released at acidic pH. This view along the α6-helix is rotated around 90° compared with (A).
(C) Close up into the α6-repulsion site (view as in [A]). Protonation of histidine and acidic residues leads to an electrostatic repulsion of the clustered positively charged residues, which subsequently destabilizes the α6-helix.
(D) pH-Dependent electrostatics of the α6-repulsion site. Red, negative potential; blue, positive potential. Contouring was set from −10 to +10. Red or blue diamonds represent R355 or E220, respectively. The arrow indicates the helix dipole moment, which will have presumably only a minor contribution to the electrostatic regulation.