Short abstract
Multiple sclerosis (MS) and systemic lupus erythematous (SLE) are autoimmune diseases, the coexistence of which is uncommon in patients. Owing to the rarity of this condition, the distinction between MS and SLE is a diagnostic challenge for neurologists. We present a case report in which MS and SLE were present in the same patient. There are few case reports in the world on the association between MS and SLE. The following case report is the first of its kind in which both MS and SLE are present in a patient from a country with low prevalence of MS such as Ecuador.
Keywords: Multiple sclerosis, systemic lupus erythematosus, Ecuador
Background
Both multiple sclerosis (MS) and systemic lupus erythematous (SLE) are autoimmune diseases.1 MS is caused by immune cell infiltration across the blood-brain barrier, which promotes inflammation, demyelination, gliosis and neuroaxonal degeneration of the white matter in the central nervous system (CNS).2,3 SLE is a B-cell-mediated autoimmune disease characterized by the generation of autoantibodies against nuclear antigens and a type III hypersensitivity leading to chronic systemic inflammation and tissue damage of various organs and systems.1,4 Genetic and environmental factors could have a role in the development of these diseases but the etiology is still unknown.1 The presence of both diseases in the same patient is rare, which suggests a relative incompatibility between these diseases.5
The prevalence of MS in the world is not homogeneous. It is higher in North American and European countries (> 100 per 100,000 inhabitants) than in South American countries such as Ecuador, in which the prevalence of MS is 1.2 per 100,000 inhabitants.6 The prevalence of SLE varies considerably worldwide. In North America, Europe and Asia the prevalence is 52, 28–71 and 30–60 cases per 100,000 inhabitants, respectively. Thus far, however, there have been no epidemiological studies on the prevalence of SLE in Ecuador.
The diagnosis of MS in a patient with SLE can be a challenge as the association between MS and SLE is rare, and at present, only 17 case reports have been written in the world. Fanouriakis et al.2 have reported the largest number of case reports with nine patients and in Latin America only one case report has been written.7 For this reason, this case report will be the second in Latin America and the first in Ecuador.
Case report
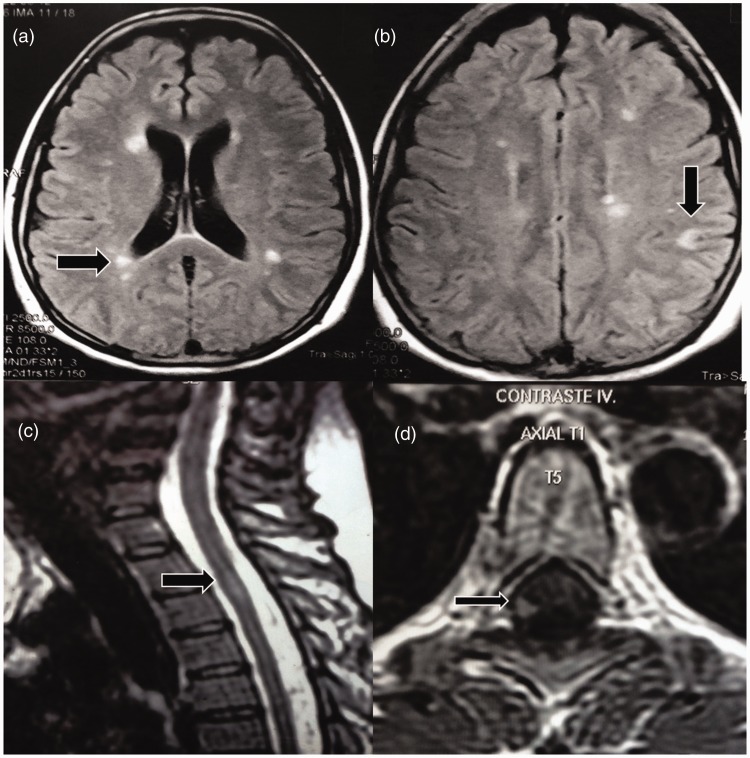
We present a 35-year-old woman with no relevant previous diseases or family history of autoimmune disorders. At the age of 32, the patient was diagnosed with relapsing–remitting MS (RRMS) according to McDonald 2010 diagnosis criteria.8 This diagnosis was established after paresis and numbness in her right foot and urinary retention was present at age 27, optic neuritis (ON) at age 29 and finally right hemiparesis at age 31. At the time of her diagnosis with RRMS, cerebrospinal fluid (CSF) oligoclonal bands (OCBs) were present. Notably, serum immunological tests, including serum antinuclear antibodies (ANA), anti-double-stranded DNA (anti-dsDNA) antibodies, anti-Ro/SSA and anti-La/SSB antibodies, antiphospholipid antibodies (aPL), human immunodeficiency virus (HIV) and Venereal Disease Research Laboratory (VDRL) tests were negative. Also, lactate dehydrogenase (LDH) and vitamin B12 levels were normal. Brain magnetic resonance imaging (MRI) showed multiple focal T2-weighted periventricular, juxtacortical and cerebellar hyperintensities. A spinal cord MRI showed one thoracic gadolinium-enhanced lesion (Figure 1). The Expanded Disability Status Scale (EDSS) was 2. The differential diagnosis of this case was conducted through the search for clinical and paraclinical red flags, which in this case were not present.
Figure 1.
Brain magnetic resonance imaging (MRI). Axial fluid-attenuated inversion recovery image reveals plaques of demyelination in the white matter (a); one left juxtacortical lesion is seen (b). Spinal cord MRI. An enhancement lesion is seen in the right lateral white matter of the spinal cord ((c) and (d)). All lesions are indicated by arrows.
The patient had been treated with intravenous methylprednisolone for MS relapses resulting in complete recovery. At age 32, the patient started treatment with subcutaneous interferon (INF)-beta-1a, which she received for three years and no evidence of disease activity was seen. At age 35, the patient chose to stop treatment with IFN as she desired to become pregnant. Six months later, the patient was diagnosed with dengue and recovered completely within a week. However, one week after her recovery from dengue, she complained of asthenia, myalgia, arthralgia, hematuria, and fever. Generalized adenopathies were found in the physical exam and laboratory investigations showed anemia (hemoglobin 9.9 g/l), leukopenia (3.5 × 109/l), lymphopenia (0.68 × 109/l), positive direct Coombs and high LDH (666 U/ml) levels. Other laboratory findings, including renal function tests and thyroid hormones, were normal. However, proteinuria (300 mg/24 h) and C3 and C4 low complement levels were identified. Virologic tests were normal or negative, ANA titers were > 7.5 U/ml (<1.2 U/ml) and anti-dsDNA titers were 1/320 (<1/10). Anti-Ro/SSA antibodies were negative. Echocardiogram showed a mild, pericardial effusion. Based on the existence of hemolytic anemia, lymphopenia, leukopenia, serositis, positive ANA and anti-dsDNA antibodies, the diagnosis of SLE was established according to the American College of Rheumatology revised criteria for the classification of SLE. The patient started treatment with hydroxychloroquine and prednisone, which were prescribed by a rheumatologist, and a new brain MRI did not show new T2 or enhancing lesions. After three months, an SLE reactivation was diagnosed after an increase of proteinuria (2.9 gr/24 h) and complement consumption was observed. Following this diagnosis, a kidney biopsy was performed that showed proliferative glomerulonephritis with class III sclerotic lesions. As a result of the findings, a multidisciplinary evaluation by neurologists, nephrologists and rheumatologists was carried out. The consensus reached was that (a) the patient had active SLE, (b) the MS was inactive from the beginning of treatment with IFN despite treatment suspension for a period of nine months, (c) rituximab was the treatment to be administered and (d) there would be monthly follow-ups of the SLE for three months, after which there would be a trimonthly follow-up and in the case of MS, after six months of rituximab administration, a cerebral MRI would be performed. After the above-mentioned six months, no further neurologic relapse symptoms were noted and brain MRI did not show any additional lesions compared with images taken from the patient at age 32 and SLE was quiescent.
Discussion
In this case report, it is important to determine if the neurologic manifestations and the brain and spinal cord lesions in MRI were due to SLE, or a result of RRMS with post-development of typical systemic manifestations of SLE due to the fact that neurological manifestations in SLE can be present years before the systemic manifestations.9 In SLE, aPL play a crucial role; the mechanism by which these antibodies can produce a disease similar to MS in patients with SLE includes the molecular mimetic with myelin, vasculopathy and autoimmune vasculitis.7 However, in our patient, aPL was negative at the beginning of the disease and during the systemic manifestation of SLE.
ON can be present in MS and SLE. In MS, ON is characterized by an acute or subacute course, with unilateral or bilateral impairment of vision and retro-orbital or ocular pain that is usually exacerbated by eye movement; a total or partial recovery follows these clinical characteristics. ON in SLE is rare; however, the characteristic of ON is an acute visual impairment that is followed by progressive visual loss lasting weeks after the initial visual impairment.10
Myelitis in MS is asymmetrical, progresses in hours or days and sphincter impairment is usually present. Myelitis in SLE is usually the first neurologic manifestation in around 21% of cases. Extensive longitudinal spinal cord damage is seen in 71% of patients and spinal cord swelling is seen in 91.7% of cases when myelitis affects gray matter. In SLE, there is a clear association between myelitis and lupus anticoagulants, both of which were negative in our patient.11
Brain MRIs in patients with SLE show focal and punctiform lesions in white matter as well as brain cortical atrophy and small-vessel disease. On the other hand, in MS, the brain lesions on MRI are ovoid and periventricular and the corpus callosum is frequently affected; it is also common to see brainstem, subcortical and spinal cord lesions.7 In our patient, the MRI lesions were similar to MS and they satisfied the Barkhof-Tintoré criteria,12 and the lack of systemic signs and the absence of ANA and anti-dsDNA for six years after onset of the first symptoms virtually excluded SLE during that period. The characteristic differences between MS and neuro-SLE are presented in Table 1.
Table 1.
| Variable | MS | Neuro-SLE |
|---|---|---|
| Optic neuritis | Present and usually unilateral | Rare |
| Spinal cord lesions | Short segment Less than half of spinal cord diameter Nodular/homogeneous enhancement Over time may become less evident |
Longitudinal extensive Predilection for central cord |
| Brain | DIS Periventricular: Perivenular, perpendicular to ventricle Thalamus/hypothalamus uncommon Brainstem: Dorsal but also pial surface/intra-axial trigeminus Cortical lesions are common |
Presence of cortical infarcts or lacunae, microhemorrhages, calcifications Predominance of lesions in the corticosubcortical junction, sometimes crossing vascular territories White-matter lesions sparing the U-fibers Punctiform parenchymal lesions. Involvement of the basal ganglia Brain atrophy may develop |
| Oligoclonal bands (CSF) | Present in >90% | Present in 15% to 50% |
| CSF | Usually normal | Usually abnormal |
| ANA | Negative or low (1:80 to 1:160) | Positive or low (>1:160) |
| Anticardiolipin antibodies | Usually negative Positive: atypical cases |
Usually positive |
| Extraneurologic manifestations | Absent | Present |
| Brain biopsy | Inflammatory demyelination | Ischemic-vasculitis-necrosis and demyelination |
MS: multiple sclerosis; SLE: systemic lupus erythematous; DIS: dissemination in space; ANA: antinuclear antibodies; CSF: cerebrospinal fluid.
In our case, the diagnosis of MS was based on the McDonald 2010 diagnosis criteria, which do not consider the presence of OCBs for the diagnosis of RRMS.8 Our patient met criteria for dissemination in time and space (DIS) despite having positive OCBs, which, at that time, was not taken into consideration in the diagnosis. However, in recent years, OCBs have begun to play a fundamental role in patients with clinically isolated syndrome (CIS) and MS.13 In this regard, a meta-analysis has shown that the presence of OCBs in patients with CIS predicts the conversion to clinically defined MS (CDMS) and this meta-analysis showed that the presence of OCBs in patients with MS was an indicator of disability progression measured by EDSS.14 A prospective study in 415 patients with CIS showed that the presence of OCBs was associated with the conversion to CDMS, and the presence of OCBs increased the risk of a second relapse.15 Arrambide et al. demonstrated that the presence of OCBs together with DIS could be an additional criterion for the diagnosis of MS in patients with CIS, which allowed the OCBs to be considered in the new McDonald 2017 diagnostic criteria.13,16 For this reason, we recommend testing for OCBs in patients with CIS since the presence of OCBs allows an earlier MS diagnosis and could be a useful predictor of disability.
MS and SLE are rarely reported to coexist in a single patient, and at present 17 cases have been reported. In patients with MS and SLE, myelitis (14/17) and ON (5/17) were the more frequent clinical manifestations of MS, which were present in our case. Arthritis (15/17) and dermic manifestations (9/17) were the most frequent systemic manifestations. This is in contrast to our report, in which renal and hematological symptoms were present. ANA and anti-dsDNA were positive in 13/17 patients (Table 2).2,5,7,9,17,18 Fanouriakis et al.2 have shown that RRMS was commonly associated with SLE in 8/9 patients, and 4/9 patients had MS before SLE, which is similar to our case.
Table 2.
Clinical characteristics of SLE-MS patients.
| Patient | Age at diagnosis of SLE/MS | SLE manifestations | MS manifestations | Therapy for SLE | Therapy for MS | |
|---|---|---|---|---|---|---|
| Fanouriakis et al.2 Greece 2014 |
1 | 40/56 | Photosensitivity, arthritis, leukopenia, ANA (+) SLICC/ACR 4 | Spinal (RRMS) | Hydroxychloroquine + azathioprine | Natalizumab |
| 2 | 44/21 | Photosensitivity, malar rash, arthritis, mouth ulcers, anticardiolipin and antiphospholipid antibodies (+) | Spinal (RRMS) | Hydroxychloroquine + azathioprine | Interferon β | |
| 3 | 36/40 | Photosensitivity, arthritis, pericarditis, mouth ulcers, ANA (+), SLICC/ACR 5 | Spinal (RRMS) | Hydroxychloroquine + azathioprine + methotrexate | Interferon β and rituximab | |
| 4 | 34/39 | Photosensitivity, malar rash, arthritis, hair loss. Antiphospholipid antibodies (+), beta-2 glycoprotein antibodies (+) | Spinal (RRMS) | Hydroxychloroquine | Interferon β | |
| 5 | 55/57 | Photosensitivity, arthritis, oral ulcers, ANA (+), SLICC/ACR 4. | Sensory-Motor (RRMS) | Hydroxychloroquine + corticosteroids | Corticosteroids | |
| 6 | 56/60 | Photosensitivity, rash malar, arthritis, ANA (+). | Spinal | Hydroxychloroquine | Corticosteroids, azathioprine, glatiramer acetate | |
| 7 | 36/34 | Photosensitivity, malar rash, chronic urticaria, arthritis, ANA (+), complement consumption, SLICC/ACR 4 | Spinal (PPMS) | Hydroxychloroquine + azathioprine | Interferon β | |
| 8 | 42/36 | Photosensitivity, arthritis, leukopenia, ANA (+), SLICC/ACR 4 | Optic neuritis (RRMS) | Hydroxychloroquine | Glatiramer acetate | |
| 9 | 35/30 | Photosensitivity, rash malar, arthritis, ANA (+). Complement consumption. SLICC/ACR 4 | Spinal (RRMS) | Hydroxychloroquine | Interferon β | |
| Kinnunen et al.9 Scandinavia 1993 |
10 | 42/30 | Pleuritis, hematuria, leukopenia, arthritis, ANA (+) | Sensory-motor Optic neuritis (RRMS) |
Corticosteroids | NA |
| 11 | 8/30 | Pleuritis, glomerulonephritis, arthritis, photosensitivity, lymphopenia, ANA (+), anti-dsDNA (+) | Peripheral facial paralysis, monoparesis MII, paraparesis, hyperreflexia, optic neuritis, seizures (RRMS) | NA | NA | |
| 12 | 57/29 | Arthritis, ANA (+), anti-dsDNA (+), complement consumption | Recurrent optic neuritis, sphincter involvement, paresis, fatigue, ataxia (RRMS) | NA | NA | |
| Hietaharju et al.17 Scandinavia 2001 |
13 | 30/18 | Arthralgias, oral ulcers, fever. ANA (+) and anti-dsDNA (+) | Spinal (PPMS) | Hydroxychloroquine | Any |
| 14 | 26/21 | Arthritis, thrombocytopenia, ANA (+) and anti-dsDNA (+) | Sensory-motor (PPMS) | NA | NA | |
| Kyrozis et al.5 Greece 2007 |
15 | 32/14 | Arthritis, erythema malar, ANA (+) and anti-dsDNA (+) | Sensory-motor (RRMS) | Hydroxychloroquine + corticosteroids and ASA | Patient refused to receive treatment. |
| Medina et al.7 Colombia 2010 |
16 | 18/16 | Polyarthralgia, hair loss, ANA + | Optic neuritis (RRMS) | Corticosteroids | NA |
| Bonaci-Nikolic et al.18 Serbia 2009 |
17 | 30/41 | Arthritis, facial edema, myalgia, fever, anemia, leukopenia, high LDH, ANA (+), anti-dsDNA (+). | Vertigo, leg numbness Myelitis (RRMS) |
Prednisone | Interferon β |
| Sánchez et al. Ecuador Present study |
18 | 33/30 | Fever, adenopathy, hematuria, proteinuria, pancytopenia, serositis, Coombs positive, high LDH, consumption complement, ANA + | Spinal (RRMS) | Hydroxychloroquine + corticosteroids | Corticosteroids IV + interferon β Currently on rituximab |
SLE: systemic lupus erythematosus; IV: intravenous; MS: multiple sclerosis; RRMS: relapsing–remitting multiple sclerosis; PPMS: primary progressive multiple sclerosis; ANA: antinuclear antibodies; SLICC/ACR: Systemic Lupus International Collaborating Clinics/American College of Rheumatology; NA: not applicable; LDH: lactate dehydrogenase; anti-dsDNA: anti-double-stranded DNA.
Our patient received subcutaneous INF beta-1a three times a week; this treatment was chosen because INF beta-1a has demonstrated efficacy through phase III clinical trials,19 and it was the only medication available in Ecuador for the treatment of RRMS. Regarding the IFNs, in patients with SLE, type I INFs have been shown to promote the activation of the immune system and alter regulatory mechanisms, contributing to inflammation and tissue damage.20 Drug-induced SLE is defined as a lupus-like syndrome related to continuous drug exposure that resolves after discontinuation of the offending drug.21 However, few case reports have shown the development of SLE in patients with MS treated with INF.22–24 This contrasts with what happened in our patient, since the symptoms of SLE were present when the medication was withdrawn and worsened despite receiving treatment with hydroxychloroquine. We believe that previous infection with the dengue virus could have triggered the expression of type I INF and the subsequent development of SLE as demonstrated in studies in which SLE developed in individuals who were exposed to live virus vaccines.20,22,23 Additionally, IFN beta has been shown to induce the death of podocytes and prevents their differentiation from their precursors, making this treatment a contraindication for patients with lupus nephritis.20
At present, there are very few therapies available for the concomitant treatment of SLE and MS. Management of SLE often depends on disease severity and disease manifestations (CNS involvement and diffuse proliferative renal disease). Hydroxychloroquine together with nonsteroidal anti-inflammatory drugs and analgesics are recommended in SLE with mild activity; prednisone together with methotrexate, azathioprine or mycophenolate mofetil (MMF) are recommended in SLE with moderate activity; and, in patients with severe activity but without renal damage or CNS involvement, cyclophosphamide, leflunamide or the combination of prednisone with MMF or rituximab are recommended.25 In class III SLE glomerulonephritis, as in the case of our patient, an induction therapy based on methylprednisolone is required together with cyclophosphamide or MMF followed by maintenance therapy based on MMF, azathioprine or cyclophosphamide in low doses. 26
Rituximab is recommended in SLE with severe neurological, hematological, or renal damage that does not respond to first-line treatments. A study has shown that rituximab can be an effective and well-tolerated therapeutic option for refractory lupus nephritis.26–28 In MS, the immunosuppressants MMF, azathioprine, methotrexate and cyclophosphamide have been studied; however, their efficacy is not yet well established. A retrospective study has shown that 55% of patients had no evidence of disease activity when followed up with cyclophosphamide as induction therapy.29 Another retrospective study showed that MMF reduced annualized relapse rate and EDSS remained stable between initiation and one year after the beginning of MMF.30 A multicenter, randomized, non-inferiority trial has shown that efficacy with azathioprine was not inferior to that of IFN beta for patients with RRMS.31 However, it is necessary that the efficacy of these drugs be demonstrated in phase III clinical trials and, if possible, be compared with disease-modifying therapies (DMTs).
Adrenocorticotropic (ACTH) hormone gel was approved by the United States Food and Drug Administration as a treatment for relapsing MS in 1978 and a treatment option for SLE in 1952.32,33 ACTH has anti-inflammatory and immunomodulatory effects due to activation of central and peripheral melanocortin receptors.34 In MS, a systematic review demonstrated that ACTH or corticosteroids were effective over the short term in improving symptoms, thus favoring recovery.35 With regard to SLE patients with moderate or severe active SLE, an open-label study showed that ACTH gel may provide significant disease activity reduction.33 Another retrospective study has shown that ACTH appears to be safe and well tolerated after six months of SLE treatment with significant reduction of disease activity.36
Our patient received treatment with rituximab, the efficacy of which in MS has been shown in observational and phase II studies. Hauser et al. have shown that compared with placebo, rituximab reduced inflammatory brain lesions and clinical relapses for 48 weeks.37 Spelman et al. have shown that rituximab was superior to first-generation DMTs with respect to relapse control and tolerability.22 An observational study showed that the rate of clinical relapses or neuroradiologic disease activity was significantly lower for rituximab when compared with injectable DMTs and dimethyl fumarate, with a tendency for a lower rate of relapses; this seems to also be the case when compared with natalizumab and fingolimod.38 Our patient had stable RRMS and she received IFN before switching to rituximab. On this point, an open-label phase II multicenter study showed that in patients with stable RRMS, a treatment switch from INF or glatiramer acetate to rituximab was associated with reduction of disease activity measured by MRI and levels of CSF neurofilament light chain.39 Also, rituximab seems to have improved effectiveness and tolerability compared with fingolimod in stable RRMS patients who switch from natalizumab because of JC virus antibody positivity.40 Finally, an observational study has shown that rituximab was safe and effective in patients with RRMS who failed to respond to first- and second-line therapies and also a useful option for patients with concomitant autoimmune disorders such as in our case report.41
In conclusion, the distinction between MS and SLE is a diagnostic challenge for the neurologist, and the presence of both diseases should be considered in patients with clinical neurologic manifestations of MS who present with typical systemic manifestations of SLE.
Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Huang J, Yang Y, Liang Z, et al. Association between the CD24 Ala57Val polymorphism and risk for multiple sclerosis and systemic lupus erythematosus: A meta-analysis. Sci Rep 2015; 5: 9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fanouriakis A, Mastorodemos V, Pamfil C, et al. Coexistence of systemic lupus erythematosus and multiple sclerosis: Prevalence, clinical characteristics, and natural history. Semin Arthritis Rheum 2014; 43: 751–758. [DOI] [PubMed] [Google Scholar]
- 3.Dendrou CA Fugger L andFriese MA.. Immunopathology of multiple sclerosis. Nat Rev Immunol 2015; 15: 545–558. [DOI] [PubMed] [Google Scholar]
- 4.Gottschalk TA Tsantikos E andHibbs ML.. Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus. Front Immunol 2015; 6: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyrozis A, Kararizou E, Georgila E, et al. Rare association of multiple sclerosis and systemic lupus erythematosus. Lupus 2007; 16: 991–992. [DOI] [PubMed] [Google Scholar]
- 6.Correa E Paredes V andMartínez B.. Prevalence of multiple sclerosis in Latin America and its relationship with European migration. Mult Scler J Exp Transl Clin 2016; 2: 2055217316666407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina YF, Martínez JB, Fernández AR, et al. Association between systemic lupus erythematosus and multiple sclerosis: Lupoid sclerosis. Revista Colombiana de Reumatología 2010; 17: 111–122. [Google Scholar]
- 8.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinnunen E, Müller K, Keto P, et al. Cerebrospinal fluid and MRI findings in three patients with multiple sclerosis and systemic lupus erythematosus. Acta Neurol Scand 1993; 87: 356–360. [DOI] [PubMed] [Google Scholar]
- 10.de Andrade FA, Guimarães Moreira Balbi G, Bortoloti de Azevedo LG, et al. Neuro-ophthalmologic manifestations in systemic lupus erythematosus. Lupus 2017; 26: 522–528. [DOI] [PubMed] [Google Scholar]
- 11.Birnbaum J, Petri M, Thompson R, et al. Distinct subtypes of myelitis in systemic lupus erythematosus. Arthritis Rheum 2009; 60: 3378–3387. [DOI] [PubMed] [Google Scholar]
- 12.Sastre-Garriga J, Tintoré M, Rovira A, et al. Specificity of Barkhof criteria in predicting conversion to multiple sclerosis when applied to clinically isolated brainstem syndromes. Arch Neurol 2004; 61: 222–224. [DOI] [PubMed] [Google Scholar]
- 13.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 14.Dobson R, Ramagopalan S, Davis A, et al. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: A meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry 2013; 84: 909–914. [DOI] [PubMed] [Google Scholar]
- 15.Tintoré M, Rovira A, Río J, et al. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology 2008; 70: 1079–1083. [DOI] [PubMed] [Google Scholar]
- 16.Arrambide G, Tintoré M, Espejo C, et al. The value of oligoclonal bands in the multiple sclerosis diagnostic criteria. Brain Epub ahead of print 16 February 2018. DOI: 10.1093/brain/awy006. [DOI] [PubMed]
- 17.Hietaharju A, Peltola J, Seppä J, et al. The coexistence of systemic lupus erythematosus and multiple sclerosis in a mother and daughter. Scand J Rheumatol 2001; 30: 120–122. [DOI] [PubMed] [Google Scholar]
- 18.Bonaci-Nikolic B, Jeremic I, Andrejevic S, et al. Anti-double stranded DNA and lupus syndrome induced by interferon-beta therapy in a patient with multiple sclerosis. Lupus 2009; 18: 78–80. [DOI] [PubMed] [Google Scholar]
- 19.Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998; 352: 1498–1504. [PubMed] [Google Scholar]
- 20.Crow MK. Advances in understanding the role of type I interferons in systemic lupus erythematosus. Curr Opin Rheumatol 2014; 26: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vedove CD, Del Giglio M, Schena D, et al. Drug-induced lupus erythematosus. Arch Dermatol Res 2009; 301: 99–105. [DOI] [PubMed] [Google Scholar]
- 22.Spelman T, Frisell T, Piehl F, et al. Comparative effectiveness of rituximab relative to IFN-β or glatiramer acetate in relapsing–remitting MS from the Swedish MS registry. Mult Scler Epub ahead of print 1 June 2017. DOI: 10.1177/1352458517713668. [DOI] [PubMed]
- 23.Sladkova V, Mares J, Lubenova B, et al. Drug-induced systemic lupus erythematosus in interferon beta-1b therapy. Neuro Endocrinol Lett 2011; 32: 4–6. [PubMed] [Google Scholar]
- 24.Bahri DM, Khiari H, Essouri A, et al. Systemic lupus erythematosus induced by interferon b1 therapy in a patient with multiple sclerosis. Fundam Clin Pharmacol 2012; 26: 210–211. [DOI] [PubMed] [Google Scholar]
- 25.Lisnevskaia L Murphy G andIsenberg D.. Systemic lupus erythematosus. Lancet 2014; 384: 1878–1888. [DOI] [PubMed] [Google Scholar]
- 26.Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012; 64: 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies RJ, Sangle SR, Jordan NP, et al. Rituximab in the treatment of resistant lupus nephritis: Therapy failure in rapidly progressive crescentic lupus nephritis. Lupus 2013; 22: 574–582. [DOI] [PubMed] [Google Scholar]
- 28.Trujillo-Martín MM, Rúa-Figueroa Fernández de Larrinoa I, Ruíz-Irastorza G, et al. Clinical practice guidelines for systemic lupus erythematosus: Recommendations for general clinical management [article in Spanish]. Med Clin (Barc) 2016; 146: 413.e1–413.e14. [DOI] [PubMed] [Google Scholar]
- 29.Harrison DM, Gladstone DE, Hammond E, et al. Treatment of relapsing–remitting multiple sclerosis with high-dose cyclophosphamide induction followed by glatiramer acetate maintenance. Mult Scler 2012; 18: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michel L, Vukusic S, De Seze J, et al. Mycophenolate mofetil in multiple sclerosis: A multicentre retrospective study on 344 patients. J Neurol Neurosurg Psychiatry 2014; 85: 279–283. [DOI] [PubMed] [Google Scholar]
- 31.Massacesi L, Tramacere I, Amoroso S, et al. Azathioprine versus beta interferons for relapsing–remitting multiple sclerosis: A multicentre randomized non-inferiority trial. PLoS One 2014; 9: e113371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berkovich RR. Acute multiple sclerosis relapse. Continuum (Minneap Minn) 2016; 22: 799–814. [DOI] [PubMed] [Google Scholar]
- 33.Fiechtner JJ andMontroy T.. Treatment of moderately to severely active systemic lupus erythematosus with adrenocorticotropic hormone: A single-site, open-label trial. Lupus 2014; 23: 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkovich R andAgius MA.. Mechanisms of action of ACTH in the management of relapsing forms of multiple sclerosis. Ther Adv Neurol Disord 2014; 7: 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Citterio A, Ciucci G, Candelise L.et al. Corticosteroids or ACTH for acute exacerbations in multiple sclerosis. Cochrane Database Syst Rev 2010; 1: CD001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Golubovsky J, Hui-Yuen J, et al. Adrenocorticotropic hormone gel in the treatment of systemic lupus erythematosus: A retrospective study of patients. F1000Res 2015; 4: 1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med 2008; 358: 676–688. [DOI] [PubMed] [Google Scholar]
- 38.Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol 2018; 75: 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Flon P, Gunnarsson M, Laurell K, et al. Reduced inflammation in relapsing–remitting multiple sclerosis after therapy switch to rituximab. Neurology 2016; 87: 141–147. [DOI] [PubMed] [Google Scholar]
- 40.Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol 2016; 79: 950–958. [DOI] [PubMed] [Google Scholar]
- 41.Berenguer-Ruiz L, Sempere AP, Gimenez-Martinez J, et al. Rescue therapy using rituximab for multiple sclerosis. Clin Neuropharmacol 2016; 39: 178–181. [DOI] [PubMed] [Google Scholar]