Figure 6.
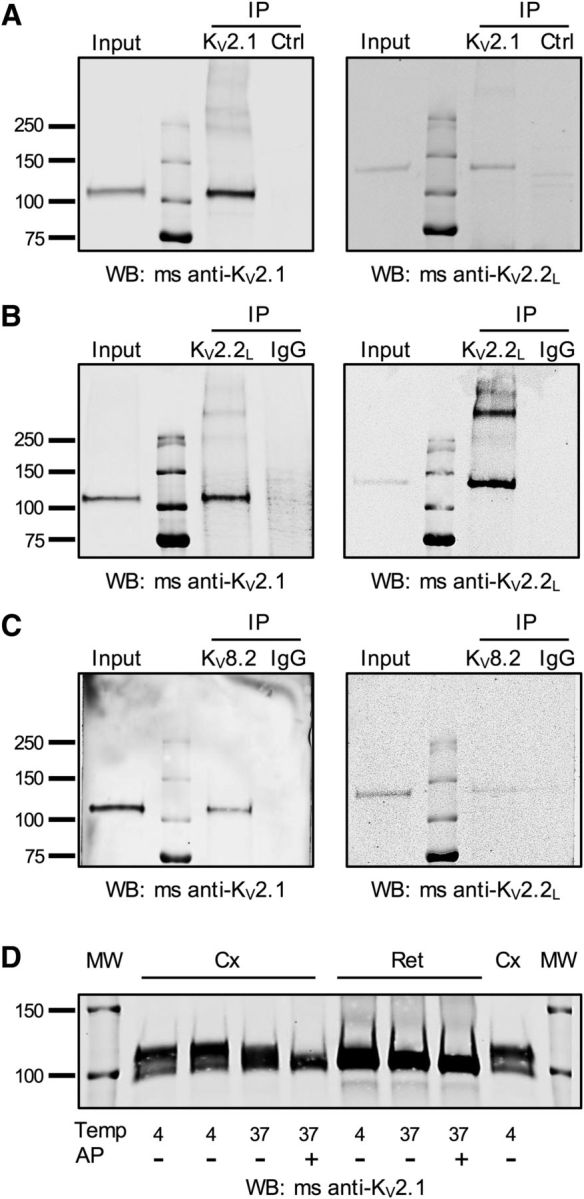
KV2 and KV8.2 subunits form heteromeric complexes. A–C, Macaque retinal lysates were subjected to immunoprecipitation with KV2.1 (A), KV2.2L (B), or KV8.2 (C) antibodies. Control experiments were performed either with normal rabbit IgG (B, C, IgG) or with an unrelated antibody (A, Ctrl, mouse anti-VGAT). Precipitated proteins were detected by Western blotting (WB) with the antibodies indicated below each blot. KV2.1 antibodies immunoprecipitated KV2.1 and coimmunoprecipitated KV2.2long (A). KV2.2long antibodies immunoprecipitated KV2.2 long and coimmunoprecipitated KV2.1 (B). In addition to the KV2.1 and KV2.2long monomers at ∼110 and ∼130 kDa, additional heavier bands were detectable with both antibodies, suggesting detection of non-reduced heteromeric channels. C, KV8.2 antibodies coimmunoprecipitate both the KV2.1 and KV2.2long subunits. D, Example of mouse neocortical (Cx) or retinal (Ret) protein lysates probed by Western blot for KV2.1. Samples were treated at temperatures (Temp) of 4°C or 37°C in the presence or absence of AP. Note the larger electrophoretic shift in cortical lysates compared with retinal lysates with AP treatment. Similar results were obtained in tissues from 4 animals. Molecular weight (MW) markers are shown on outside lanes.
