Figure 1. Enrollment, Randomization, and Follow-up of the Participants.
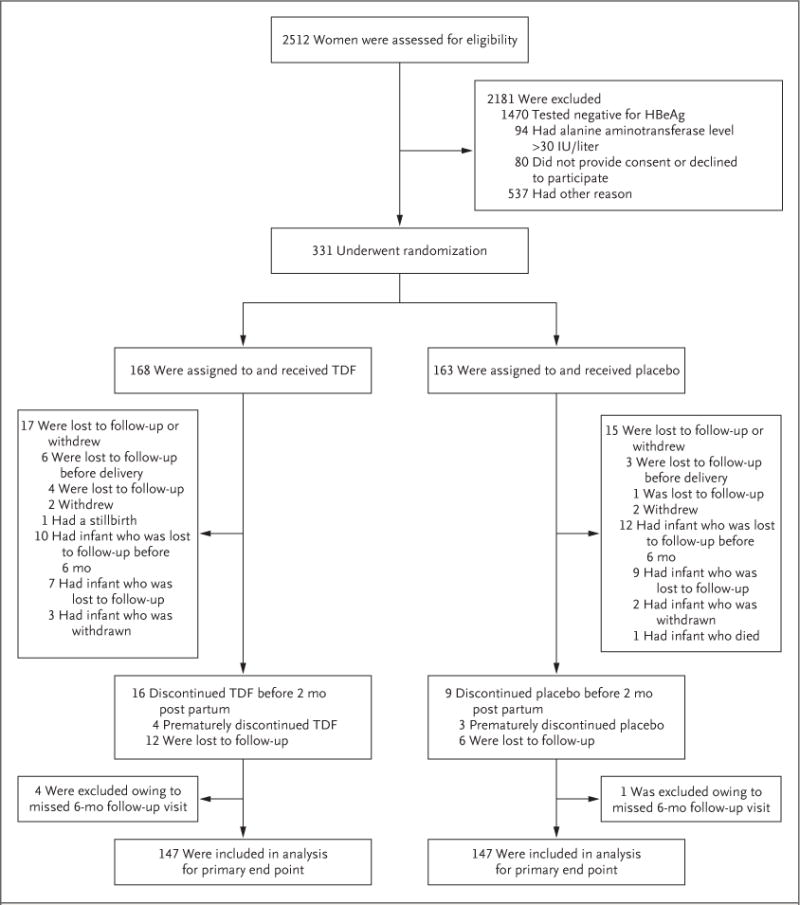
Other reasons for exclusion from the trial included the following: had an infant with a gestational age of more than 28 weeks at the screening or enrollment visit; planned to be followed at a nontrial site; did not return after screening visit; was younger than 18 years of age; had a positive test for hepatitis C virus or the human immunodeficiency virus; terminated the pregnancy; had a delivery, miscarriage, fetal death, or molar pregnancy; was receiving antiviral agents; had proteinuria or another contraindication; or presented after enrollment in the trial was already complete. HBeAg denotes hepatitis B e antigen, and TDF tenofovir disoproxil fumarate.
