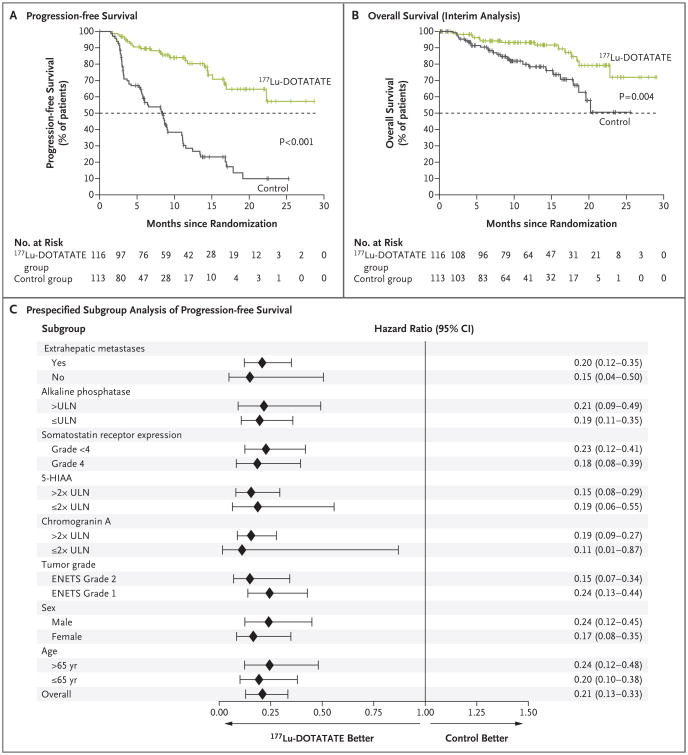
Figure 1. Progression-free Survival and Overall Survival.
Panel A shows the results of the Kaplan–Meier analysis of progression-free survival as assessed by independent central reviewers who were unaware of the treatment assignments, and Panel B the results of the planned interim analysis of overall survival. Tick marks in Panel A represent data censored at the last time the patient was known to be alive and without disease progression and tick marks in Panel B represent data censored at the last time the patient was known to be alive. Panel C shows the effect of trial treatment on progression-free survival in prespecified subgroups. European Neuroendocrine Tumor Society (ENETS) grade 1 indicates a low-grade tumor, and ENETS grade 2 indicates an intermediate-grade tumor. The 177Lu-Dotatate group received 177Lu-Dotatate at a dose of 7.4 GBq every 8 weeks (four intravenous infusions, plus best supportive care including octreotide long-acting repeatable [LAR] administered intramuscularly at a dose of 30 mg). The control group received octreotide LAR alone administered intramuscularly at a dose of 60 mg every 4 weeks. 5-HIAA denotes 5-hydroxyindoleacetic acid, CI confidence interval, and ULN upper limit of the normal range.