Figure 1.
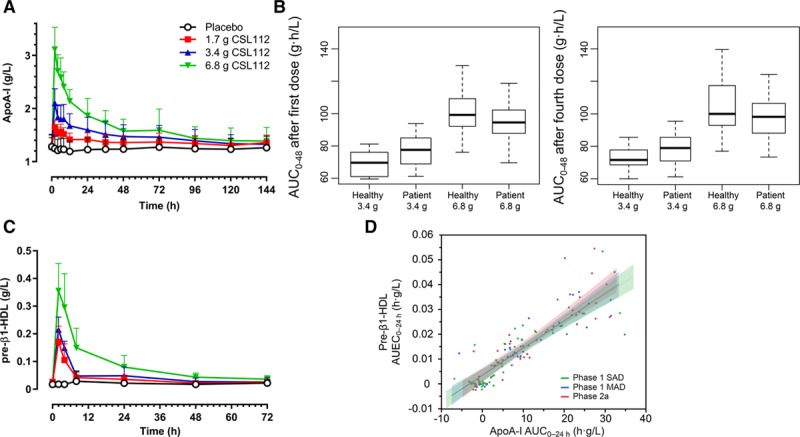
Apolipoprotein A-I (apoA-I) pharmacokinetic profile after infusion of CSL112. Infusions started at time=0 h and lasted for 2 h. A, Shown are mean+SD unadjusted total plasma apoA-I concentrations. B, Predicted exposure (area under the curve 0−48 h [AUC0−48]) in healthy subjects and patients with stable atherosclerotic disease based on population pharmacokinetic modeling. C, Shown are mean+SD unadjusted pre–β1-high-density lipoprotein (HDL) concentrations, measured by ELISA (Sekisui, Japan), in the phase 2a study. D, The relationship of baseline-corrected apoA-I exposure (AUC0−24) and baseline-corrected pre–β1-HDL exposure (area under the effect curve 0−24 h [AUEC0−24]) is shown in healthy volunteers (phase 1 single ascending dose [SAD] and multiple ascending dose [MAD] studies) and in a phase 2a study in stable atherosclerotic disease for comparison. Shown are linear regression lines and 95% CIs for each study, calculated using a random effects regression model with patient as the random effect and testing the parallelism of slopes hypothesis (difference in slopes, P=0.5).
