Abstract
To investigate the subjective and quantitative image quality and radiation exposure of CT enterography (CTE) examination performed at low tube voltage and low concentration of contrast agent with adaptive statistical iterative reconstruction (ASIR) algorithm, compared with conventional CTE.
One hundred thirty-seven patients with suspected or proved gastrointestinal diseases underwent contrast enhanced CTE in a multidetector computed tomography (MDCT) scanner. All cases were assigned to 2 groups. Group A (n = 79) underwent CT with low tube voltage based on patient body mass index (BMI) (BMI < 23 kg/m2, 80 kVp; BMI ≥ 23 kg/m2, 100 kVp) and low concentration of contrast agent (270 mg I/mL), the images were reconstructed with standard filtered back projection (FBP) algorithm and 50% ASIR algorithm. Group B (n = 58) underwent conventional CTE with 120 kVp and 350 mg I/mL contrast agent, the images were reconstructed with FBP algorithm. The computed tomography dose index volume (CTDIvol), dose length product (DLP), effective dose (ED), and total iodine dosage were calculated and compared. The CT values, contrast-to-noise ratio (CNR), and signal-to-noise ratio (SNR) of the normal bowel wall, gastrointestinal lesions, and mesenteric vessels were assessed and compared. The subjective image quality was assessed independently and blindly by 2 radiologists using a 5-point Likert scale.
The differences of values for CTDIvol (8.64 ± 2.72 vs 11.55 ± 3.95, P < .001), ED (6.34 ± 2.24 vs 8.52 ± 3.02, P < .001), and DLP (422.6 ± 149.40 vs 568.30 ± 213.90, P < .001) were significant between group A and group B, with a reduction of 25.2%, 25.7%, and 25.7% in group A, respectively. The total iodine dosage in group A was reduced by 26.1%. The subjective image quality did not differ between the 2 groups (P > .05) and all image quality scores were greater than or equal to 3 (moderate). Fifty percent ASIR-A group images provided lower image noise, but similar or higher quantitative image quality in comparison with FBP-B group images.
Compared with the conventional protocol, CTE performed at low tube voltage, low concentration of contrast agent with 50% ASIR algorithm produce a diagnostically acceptable image quality with a mean ED of 6.34 mSv and a total iodine dose reduction of 26.1%.
Keywords: adaptive statistical iterative reconstruction, computed tomography, image quality, low tube voltage, radiation dosage
1. Introduction
Gastrointestinal diseases are very common in clinic, and they have grown up to be a primary cause of mortality.[1] Many imaging modalities have been used to identify and diagnose gastrointestinal diseases. Traditional and capsule endoscopy can directly improve mucosal visualization, but these methods are unable to visualize the entire bowel wall and the extraluminal abnormalities.[2] Although MR enterography has become a standard imaging method, it is time consuming and susceptible to bowel movement and breathing, resulting in poor image quality.[3] With the higher spatial resolution, multiplanar reformation, wide scanning range, computed tomography enterography (CTE) has become the most important method for evaluating gastrointestinal disorders, particularly for small bowel diseases.[4–6] The major drawback of CT is exposure to ionizing radiation, particularly for young patients.[3] Many studies have proved that low tube voltage was a practical approach applied to reduce radiation dose during CT acquisition.[7–9] Nakaura et al[10] has also confirmed that the radiation dose decreased by 20% when the tube voltage decreased from 120 to 80 kVp. However, a principal byproduct of low tube voltage is the loss of image quality because of the associated increase in image noise and higher susceptibility to beam-hardening artifacts, especially for those patients with high body mass index (BMI).[1] The application of adaptive statistical iterative reconstruction (ASIR) algorithm has the potential to overcome the increased image noise, improve the image quality, and decrease the radiation dose,[11–13] and a 30% to 50% blending level of ASIR algorithm can produce a better image quality.[1,10]With the rapid development of multidetector computed tomography (MDCT), it has been reported that contrast-induced nephropathy (CIN) was one of the main causes of hospital-acquired acute renal failure due to the increased application of contrast-enhanced CT.[14] Hence, the smallest diagnostically appropriate amount of contrast material should be used in patients, particularly those with chronic kidney disease.[15] Several studies have investigated the clinical utility of low tube voltage and low concentrations of contrast agent (270 mg I/mL) in displaying blood vessels.[12,13] But, there are a few published studies about the application of low tube voltage combined with low concentration contrast agent to CTE. Hence, the purpose of the present study was to discuss whether it was feasible to acquire diagnostically acceptable image quality when CTE performed at low contrast agent, low tube voltage based on BMI with 50% ASIR algorithm on patients with suspected or proved gastrointestinal diseases.
2. Materials and methods
2.1. Study cohort
This study was approved by our institutional review board, and written informed consent was obtained from all patients before the examination. Patients who were suspected or proved of having gastrointestinal diseases were enrolled. Inclusion criterion was a patient who underwent CTE examination. Clinical exclusion criteria were pregnancy, allergy to the contrast medium, renal impairment, (estimated glomerular filtration rate < 60 mL/min/1.73 m2, measured within 2 weeks before the enrollment). Eleven patients were excluded for these reasons. At last, 137 patients (65 female, 72 male; mean age 54 ± 14 years; age range 19–84 years) were included from October 2015 to February 2016 in this prospective study. A flowchart of the study population is shown in Fig. 1.
Figure 1.
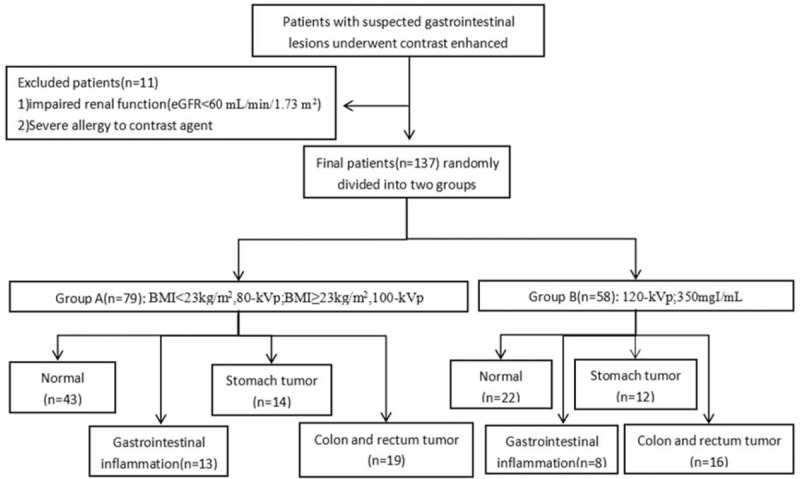
Flowchart of the study population.
2.2. CT examination
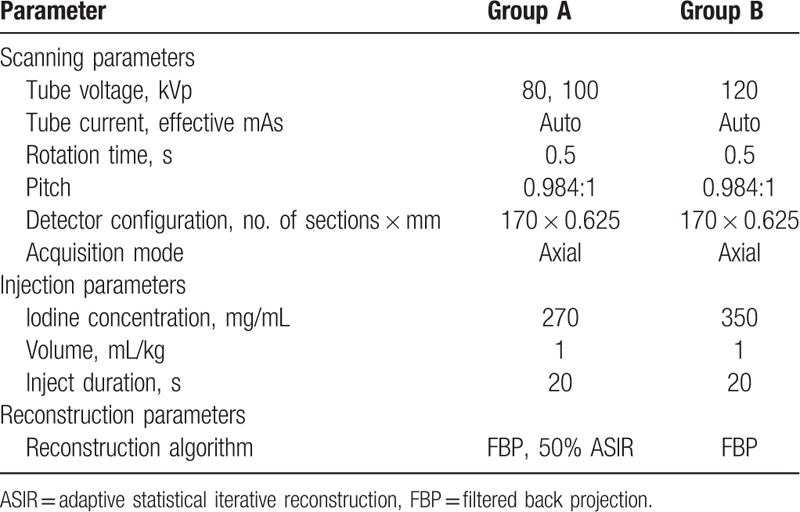
All patients were assigned to the 2 groups and underwent contrast-enhanced CTE using a 64-slice MDCT scanner (Discovery CT750 HD, GE Healthcare, Milwaukee, WI). Group A (n = 79) underwent at low tube voltage (BMI < 23 kg/m2, 80 kVp; BMI ≥ 23 kg/m2, 100 kVp), low contrast agent concentration (iodixanol, 270 mg I/mL, GE Healthcare) contrast-enhanced MDCT, and the raw data were reconstructed with filtered back projection (FBP) and 50% ASIR (GE Healthcare). Group B (n = 58) underwent at 120 kVp and conventional contrast agent concentration (Optiray, 350 mg I/mL, Mallinckrodt, Canada), and images were reconstructed with FBP.
A thorough dilatation of the bowel was required to obtain diagnostic gastrointestinal images. We chose mannitol (20% w/v; Double-Crane Pharmaceutical, Beijing, China) as the contrast agent in our study. This agent retards the resorption of water in the intestine. Our specific protocol for CTE required patients to fast for 6 hours before the procedure. The solution was prepared by diluting 250 mL of mannitol with 1750 mL of water to produce an isoosmotic solution. If the patients could tolerate, they were encouraged to ingest 1500 to 2000 mL of the solution every 10 minutes for 40 minutes to dilate the gastrointestinal tract.
All patients were placed in a supine, feet-first position on the CT couch, and the scanning volume was acquired from the top of the diaphragm to the perineum in a cephalocaudal direction during a single breath-hold to minimize motion artifacts. Dual-phase, contrast-enhanced helical images of the entire abdomen were obtained. The arterial phase (AP) scanning was initiated by bolus tracking (Smartprep, GE Healthcare Technologies, WI) when a threshold enhancement of 120 Hounsfield unit (HU) was achieved in the abdominal aorta. The venous phase (VP) was initiated 25 to 30 seconds after the completion of AP scanning. Contrast material was injected through the antecubital vein with an 18 gauge intravenous cannula using a double-tube high-pressure injector (Stellant, Medrad, CO, WI), followed by a 20 mL saline flush, each with an injection time of 20 seconds. The total contrast volume was 1 mL/kg.
The CT imaging parameters for both groups were as follows: automatic tube current; rotation time, 0.5 seconds; detector pitch, 0.984:1; matrix, 512 × 512; table speed, 39.37 mm/rotation; and slice thickness/interval, 0.625 mm (Table 1).
Table 1.
CT scanning parameters, injection parameters, and postprocessing algorithms.
All images were transferred to the workstation (ADW 4.6; GE Medical Systems, Milwaukee, WI) for quantitative and qualitative analysis.
2.3. Qualitative analysis
Two board-certified abdominal radiologists (Z.L., 14 years of experience; F.C., 6 years of experience) who were blinded to the scan technique, reconstruction methods and pathology results performed the qualitative analysis in a random order. The subjective scale used to assess image quality was the 5-point Likert scale, with 5 being the highest quality and 1 being the lowest (1 = poor; 2 = acceptable; 3 = moderate; 4 = good; 5 = excellent), which was based on the spatial resolution, soft-tissue contrast, sharpness of tissue interfaces, conspicuity of anatomical detail, degree of image degradation by streak or beam-hardening artifacts and the overall image quality. Only an image quality of 3 or higher was considered sufficient for diagnosis.
2.4. Quantitative analysis
Quantitative image analysis of each CT examination was conducted on the workstation in a blind manner. Image noise was recorded as standard deviation (SD) of the CT attenuations in a region of the subcutaneous fat of the anterior abdominal wall on axial images.[8] To minimize measurement bias, each region of interest (ROI) was measured 3 times and average data were acquired. The CT attenuations of the main superior mesentery artery (SMA), superior mesentery vein (SMV) were measured by placing a circular ROI in the center of the vessels on axial images, as described in previous studies.[8,16] CT attenuations of background were measured by setting a ROI on the erector spinae at the same layer. The attenuations of gastrointestinal tumors were measured by drawing an irregular ROI along the lesion margin on the largest cross section at the VP, excluding blood vessels, necrosis, lumen contents, and extraenteric tissues. For the inflamed bowel with wall thickening and layered enhancement, the ROIs were set within the mucosa exhibiting the greatest degree of enhancement on axial images at the VP.[17] For normal small bowel, the distended loop was selected. Mural CT attenuation at the VP was measured by placing a small enough ROI within inner-part of the bowel wall, moved it slowly and get the highest attenuation.[18] The signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were calculated as follows: SNR = CTtarget/SDbackground and CNR = (CTtarget − CTbackground)/SDbackground.
2.5. Radiation dose
The computed tomography dose index volume (CTDIvol) and dose length product (DLP) were recorded for each CT scan. The effective dose (ED) was calculated with the following equation: ED = k × DLP and k = 0.015 (mSv × mGy−1 × cm−1), which is the dose conversion factor for CT of the abdomen, according to the guidelines of the International Commission on Radiological Protection.[19]
2.6. Statistical analysis
Statistical analysis was performed with SPSS statistical software (version 18.0 for Windows). All quantitative values were expressed as mean ± SD and an unpaired Student t test was used to investigate differences in clinical data (age, height, body weight, BMI, and maximum transverse diameter), radiation dose (CTDIvol, ED, and DLP), quantitative image quality parameters (CT values, image noise, SNR, and CNR) between the group A and group B. The subjective image quality scores between the FBP-A and FBP-B groups, 50% ASIR-A and FBP-B groups were compared with Chi-square test. Kappa analysis was used to evaluate the inter-observer agreement between the 2 radiologists with κ statistics. A P-value < .05 indicated a statistically significant difference.
3. Results
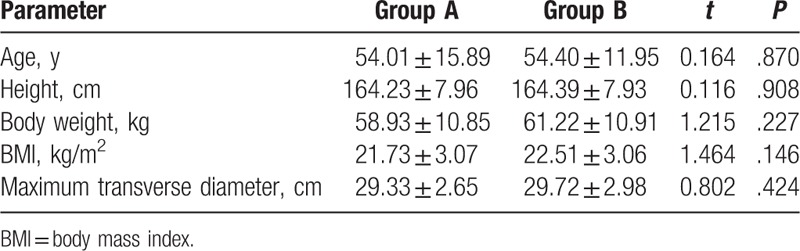
Clinical data (patient age, height, body weight, BMI, and maximum transverse diameter) are summarized in Table 2. There were no significant differences in patient age (P = .870), height (P = .908), body weight (P = .227), BMI (P = .146), and maximum transverse diameter (P = .424) between the 2 groups.
Table 2.
Comparison of the clinical data between group A and group B.
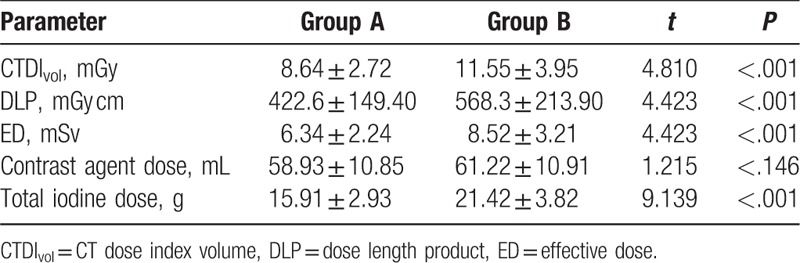
The radiation dose and contrast agent dose are shown in Table 3. The radiation dose decreased obviously with the application of low tube voltage in group A. The differences of values for CTDIvol (8.64 ± 2.72 mGy vs 11.55 ± 3.95 mGy, P < .001), ED (6.34 ± 2.24 mSv vs 8.52 ± 3.02 mSv, P < .001), and DLP (422.6 ± 149.40 mGy-cm vs 568.30 ± 213.90 mGy-cm, P < .001) were significant between the 2 groups, with a reduction of 25.2%, 25.7%, and 25.7% in group A, respectively. There was no difference in the mean contrast agent dose between the 2 groups (P = .227). The mean total iodine dosage was significantly lower in group A (15.91 ± 2.93 g) than group B (21.42 ± 3.82 g) (P < .001), with a reduction of 26.1%.
Table 3.
Comparison of the radiation dose, contrast agent dose and total iodine dose between group A and group B.
The results of qualitative analysis are summarized in Table 4. Both protocols yielded excellent subjective image quality. All image quality scores were greater than or equal to 3 (moderate) with respect to the overall image quality and enhancement of the SMA, SMV, mucosa of normal bowel, and gastrointestinal lesions. There was substantial interobserver agreement with respect to image quality (Kappa = .627). There were no significant differences between the FBP-B group and FBP-A group, FBP-B group and the 50% ASIR-A group (P = .439 and P = .157, respectively). Image noise was lower in the 50% ASIR-A group than the FBP-A and FBP-B groups (Fig. 2).
Table 4.
Comparison of qualitative analysis scores of the images of the FBP-A and 50% ASIR-A with FBP-B groups.
Figure 2.
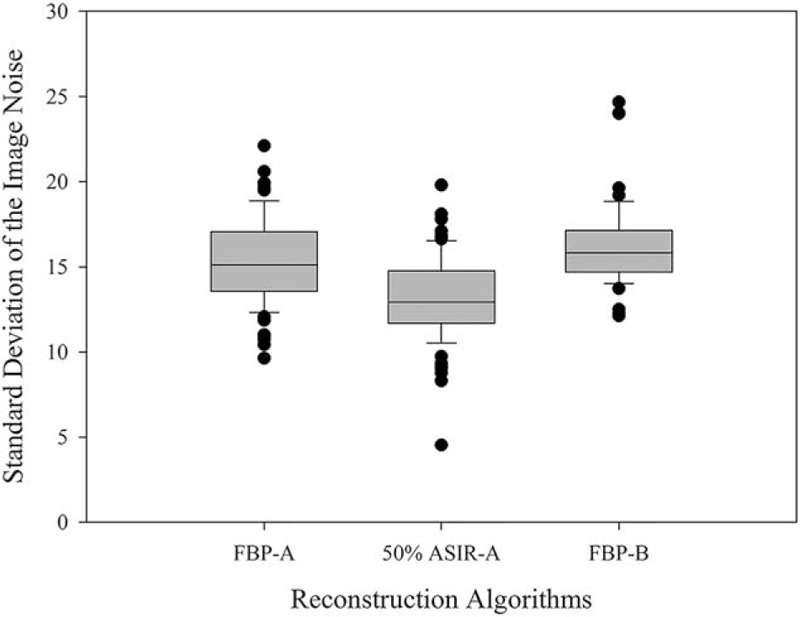
Box-and-whisker plots showing the mean and standard deviation of the image noise among the different reconstruction protocols. The noise of the images reconstructed with 50%ASIR of group A was significantly lower than the images reconstructed with FBP of group A and group B.
CT values, CNR and SNR of the FBP-A group and 50% ASIR-A group were compared with those of the FBP-B group. Compared with the FBP-B group, the CT values of the SMA, SMV, and gastrointestinal tumors in VP were significantly higher in group A (P < .05) (Table 5 and Fig. 3). The values for CNR and SNR of the SMA and SMV were significantly higher in the FBP-A group and 50% ASIR-A group than FBP-B group (P < .05). The values for CNR and SNR of the gastrointestinal tumors in the 50% ASIR-A group were significantly higher than FBP-B group (P < .05), there was no significant difference between the FBP-A and FBP-B groups (P > .05). However, there were no significant differences in CT values, CNR and SNR of mucosa of the normal and inflamed bowel between the 2 groups (P > .05).
Table 5.
Comparison of the CT values, SNR and CNR in mucosa of the normal intestine, inflammatory diseases, tumors, SMA and SMV of the FBP-A and 50% ASIR-A with FBP-B groups.
Figure 3.
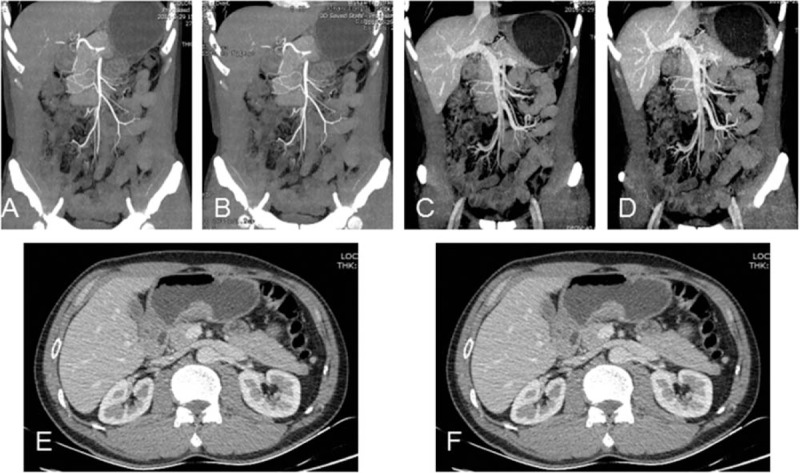
CTE of a 53-year-old male patient (BMI: 20.1 kg/m2) with gastric adenocarcinoma underwent CTE at 80 kVp/270 mg I/mL with FBP and 50%ASIR algorithm. Maximum intensity projection images of SMA reconstructed with FBP (A), 50% ASIR (B) and SMV reconstructed with FBP (C), 50% ASIR (D). Axial images show wall thickening of the gastric antrum reconstructed with FBP (E) and 50% ASIR (F) respectively. CTDIvol, DLP, ED, and total iodine dose were 7.18 mGy, 316.67 mGy-cm, 4.75 mSv, and 16.85 g. SD of the images reconstructed with 50% ASIR was reduced obviously and subjective image quality was similar between the 2 reconstruction methods.
4. Discussion
CTE is an essential modality and plays an important role in the primary diagnosis and follow-up of patients with suspected or proved gastrointestinal diseases.[6,20] The need to reduce the radiation dose of the contrast enhanced CT while maintaining image quality is generally known. As a main strategy to decrease radiation dose, lower tube voltage has been reported to be used in many areas.[12,21,22] The use of a low tube voltage of 80 kVp technique for CTE in patients with lower body weight while maintaining image quality has also been reported.[23] More studies have suggested that patient BMI was a better criterion for determining the tube voltage. Hence, in our study, we chosen different tube voltage based on patient BMI rather than body weight.
In our study, CTE performed at lower tube voltage and a lower concentration of contrast agent exhibited an approximately 25.7% reduction of ED and a 26.1% reduction of contrast agent dose, compared with CTE examinations performed at 120 kVp with FBP image reconstruction algorithm. Del Gaizo et al[24] concluded that reducing the tube voltage to 80 or 100 kVp could produce a 16% to 30% reduction of radiation dose, which was consistent with our results. However, the reduction of radiation dose reported in our study was lower than that reported by Kaza et al,[23] in which CTE was only performed at 80 kVp in patients weighing less than 160 lb and the radiation dose was decreased by 40%. The reason for the difference may be that overweight patients were not excluded from our study. Previous study has demonstrated that the image noise affected image quality when patient size exceeded a given threshold.[25] Hence, the decease of tube voltage should be based on patient size. Otherwise, because of the motion artifacts, it was more challenging for us to maintain diagnostically acceptable image quality of the gastrointestinal tract than the retroperitoneal organs in abdominal CT. Therefore, in our study patients with BMI over than 23 kg/m2 underwent CTE at 100 kVp instead of 80 kVp, which contributed to a relative increase in radiation dose.
During the quantitative analysis, our results indicated that the CT values and CNR of SMA and SMV in group A were significantly higher than those in group B. This result indicated that lower tube voltage technique combined with lower concentration of contrast agent could display blood vessels more clearly. Similar result has also been reported in the study published by Keller et al,[26] they concluded that the increased attenuation of iodine was another superiority of lower tube voltage, leading to increased tissue contrast of enhanced tissues. This increased image contrast could be beneficial in evaluating patients with suspected gastrointestinal lesions, such as inflammatory bowel diseases, in which mucosa can be obviously enhanced. In our study, the quantitative image quality of gastrointestinal tumors in the FBP-A and 50% ASIR-A groups were significantly higher than those of group B, analogous result has been reported by Marin et al,[22] they concluded that ASIR significantly improved conspicuity of hypervascular liver lesions. However, there were no statistically significant differences in CT values of the normal and the inflamed bowel wall between the 2 protocols in our study. This result was contradicted with the previous study, in which mucosal hyperenhancement are more pronounced at lower tube voltage of inflamed bowel.[27]
One possible explanation could be that the mucosa was too thin for the radiologists to set ROI in the right place within mucosa of normal intestine, which may lead to measurement bias. Otherwise, the number of cases with inflamed bowel was comparably less in our study, related research would continue in patients with inflammatory bowel disease, who need repeated imaging examinations.
Low tube voltage protocol affects image quality because of the associated increase in image noise and higher susceptibility to beam-hardening artifacts.[28] The decrease in tube voltage leads to a reduction in the amount of penetrating radiation, resulting in increased image noise, particularly in obese patients.[29] ASIR algorithm was a main noise reduction technique that used to compensate for the increased image noise in lower tube voltage examinations. Many previous studies have demonstrated that the combination of lower tube voltage with ASIR images reconstruction algorithm could reduce the radiation dose while maintaining image quality.[12,23] ASIR algorithm could be conducted at different blending levels, and we used a 50%ASIR in our study, which was used as our clinical protocol. Image noise was reflected by SNR and SD. As expected, in our study, the 50% ASIR-A group images provided lower value for SD, but similar or higher values for SNR in comparison with FBP-A group images. This result was consistent with the study published by Li et al[12] with adrenal and nephrogenic hypertension, they also demonstrated that SD values in the ASIR-groups were obviously lower than the FBP groups. Depending on the results of the quantitative image quality analysis, lower tube voltage was not thought to affect the ability to detect obvious gastrointestinal diseases, and the increase in noise could be reduced by the use of ASIR algorithm.
Regarding the subjective image quality score, all image quality scores were greater than or equal to 3 (moderate) with respect to the overall image quality and enhancement of the SMA, SMV, normal bowel wall, and gastrointestinal lesions. We concluded that the low tube voltage CTE technique had a similar subjective image quality as the 120 kVp CTE protocol for the detection of gastrointestinal disorders.
Our study has a few limitations. First, although significant differences were calculated, the population that underwent 120 kVp protocol was too small compared to the population that underwent low tube voltage protocol. Second, instead of reconstructing images with ASIR at different levels of blending, only 50% blending of ASIR instead of different percent blending was calculated in our study. Third, we focused on comparing the radiation dose, contrast agent dose and image quality, but the influence of BMI was not properly considered, which can affect the image quality obviously. The fourth limitation was that a pathological diagnosis was not given for all the gastrointestinal lesions in our sample.
In conclusion, CTE performed at lower tube voltage and lower contrast agent dose yielded diagnostically adequate image quality, with a 26.1% reduction in the contrast agent dose and a 25.7% reduction in the radiation dose, compared with the CTE performed at standard tube voltage and contrast agent dose.
5. Author contributions
Conceptualization: C. Feng, Z. Li.
Data curation: C. Feng, D. Zhu, X. Zou.
Formal analysis: C. Feng, A. Li.
Funding acquisition: Z. Li, D. Hu.
Methodology: C. Feng,. X. Hu, Z. Li.
Project administration: D. Hu.
Supervision: Z. Li, D. Hu.
Writing – original draft: C. Feng.
Writing – review & editing: Z. Li, D. Hu.
Footnotes
Abbreviations: AP = arterial phase, ASIR = adaptive statistical iterative reconstruction, BMI = body mass index, CNR = contrast-to-noise ratio, CTDIvol = computed tomography dose index volume, CTE = computed tomography enterography, DLP = dose length product, ED = effective dose, FBP = filtered back projection, HU = Hounsfield unit, MDCT = multidetector computed tomography, ROI = region of interest, SD = standard deviation, SMA = superior mesentery artery, SMV = superior mesentery vein, SNR = signal-to-noise ratio, VP = venous phase.
Funding: This study was supported by grants from the National Natural Science Foundation of China (nos. 81771801, 81271529, 81371524, 81571642).
The authors have no conflicts of interest to disclose.
References
- [1].Paulsen SR, Huprich JE, Fletcher JG, et al. CT enterography as a diagnostic tool in evaluating small bowel disorders: review of clinical experience with over 700 cases. Radiographics 2006;26:641–57. [DOI] [PubMed] [Google Scholar]
- [2].Casciani E, Masselli G, Di Nardo G, et al. MR enterography versus capsule endoscopy in paediatric patients with suspected Crohn's disease. Eur Radiol 2011;21:823–31. [DOI] [PubMed] [Google Scholar]
- [3].Towbin AJ, Sullivan J, Denson LA, et al. CT and MR enterography in children and adolescents with inflammatory bowel disease. Radiographics 2013;33:1843–60. [DOI] [PubMed] [Google Scholar]
- [4].Meng X, Ni C, Shen Y, et al. Differentiating malignant from benign gastric mucosal lesions with quantitative analysis in dual energy spectral computed tomography. Medicine 2017;96:e5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kordbacheh H, Baliyan V, Serrao J, et al. Imaging in patients with Crohnʼs disease. Inflamm Bowel Dis 2017;23:1025–33. [DOI] [PubMed] [Google Scholar]
- [6].Masselli G, Di Tola M, Casciani E, et al. Diagnosis of small-bowel diseases: prospective comparison of multi-detector row CT enterography with MR enterography. Radiology 2016;279:420–31. [DOI] [PubMed] [Google Scholar]
- [7].Kim JH, Choo KS, Moon TY, et al. Comparison of the image qualities of filtered back-projection, adaptive statistical iterative reconstruction, and model-based iterative reconstruction for CT venography at 80 kVp. Eur Radiol 2016;26:2055–63. [DOI] [PubMed] [Google Scholar]
- [8].Lv P, Liu J, Chai Y, et al. Automatic spectral imaging protocol selection and iterative reconstruction in abdominal CT with reduced contrast agent dose: initial experience. Eur Radiol 2017;27:374–83. [DOI] [PubMed] [Google Scholar]
- [9].Zhang X, Li S, Liu W, et al. Double-low protocol for hepatic dynamic CT scan. Medicine 2016;95:e4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nakaura T, Awai K, Maruyama N, et al. Abdominal dynamic CT in patients with renal dysfunction: contrast agent dose reduction with low tube voltage and high tube current-time product settings at 256-detector row CT. Radiology 2011;261:467–76. [DOI] [PubMed] [Google Scholar]
- [11].Kaul D, Grupp U, Kahn J, et al. Reducing radiation dose in the diagnosis of pulmonary embolism using adaptive statistical iterative reconstruction and lower tube potential in computed tomography. Eur Radiol 2014;24:2685–91. [DOI] [PubMed] [Google Scholar]
- [12].Li Z, Li Q, Shen Y, et al. Adrenal and nephrogenic hypertension: an image quality study of low tube voltage, low-concentration contrast media combined with adaptive statistical iterative reconstruction. Int J Clin Pract 2016;70:B29–36. [DOI] [PubMed] [Google Scholar]
- [13].Padole A, Singh S, Lira D, et al. Assessment of filtered back projection, adaptive statistical, and model-based iterative reconstruction for reduced dose abdominal computed tomography. J Comput Assist Tomogr 2015;39:462–7. [DOI] [PubMed] [Google Scholar]
- [14].Josephson SA, Dillon WP, Smith WS. Incidence of contrast nephropathy from cerebral CT angiography and CT perfusion imaging. Neurology 2005;64:1805–6. [DOI] [PubMed] [Google Scholar]
- [15].Noda Y, Kanematsu M, Goshima S, et al. Reducing iodine load in hepatic CT for patients with chronic liver disease with a combination of low-tube-voltage and adaptive statistical iterative reconstruction. Eur J Radiol 2015;84:11–8. [DOI] [PubMed] [Google Scholar]
- [16].Nakaura T, Nakamura S, Maruyama N, et al. Low contrast agent and radiation dose protocol for hepatic dynamic CT of thin adults at 256-detector row CT: effect of low tube voltage and hybrid iterative reconstruction algorithm on image quality. Radiology 2012;264:445–54. [DOI] [PubMed] [Google Scholar]
- [17].Sharman A, Zealley IA, Greenhalgh R, et al. MRI of small bowel Crohn's disease: determining the reproducibility of bowel wall gadolinium enhancement measurements. Eur Radiol 2009;19:1960–7. [DOI] [PubMed] [Google Scholar]
- [18].Wu Y, Tang Y, Hao N, et al. Crohn's disease: CT enterography manifestations before and after treatment. Eur J Radiol 2012;81:52–9. [DOI] [PubMed] [Google Scholar]
- [19].Maldjian PD, Goldman AR. Reducing radiation dose in body CT: a primer on dose metrics and key CT technical parameters. AJR Am J Roentgenol 2013;200:741–7. [DOI] [PubMed] [Google Scholar]
- [20].Mao R, Gao X, Zhu Z, et al. CT enterography in evaluating postoperative recurrence of Crohn's disease after ileocolic resection. Inflamm Bowel Dis 2013;19:977–82. [DOI] [PubMed] [Google Scholar]
- [21].Buls N, Van Gompel G, Van Cauteren T, et al. Contrast agent and radiation dose reduction in abdominal CT by a combination of low tube voltage and advanced image reconstruction algorithms. Eur Radiol 2015;25:1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Marin D, Choudhury KR, Gupta RT, et al. Clinical impact of an adaptive statistical iterative reconstruction algorithm for detection of hypervascular liver tumours using a low tube voltage, high tube current MDCT technique. Eur Radiol 2013;23:3325–35. [DOI] [PubMed] [Google Scholar]
- [23].Kaza RK, Platt JF, Al-Hawary MM, et al. CT enterography at 80 kVp with adaptive statistical iterative reconstruction versus at 120 kVp with standard reconstruction: image quality, diagnostic adequacy, and dose reduction. AJR Am J Roentgenol 2012;198:1084–92. [DOI] [PubMed] [Google Scholar]
- [24].Del Gaizo AJ, Fletcher JG, Yu L, et al. Reducing radiation dose in CT enterography. Radiographics 2013;33:1109–24. [DOI] [PubMed] [Google Scholar]
- [25].Yu L, Li H, Fletcher JG, et al. Automatic selection of tube potential for radiation dose reduction in CT: a general strategy. Med Phys 2010;37:234–43. [DOI] [PubMed] [Google Scholar]
- [26].Keller MR, Kessler RM, Brooks RA, et al. Optimum energy for performing CT iodinated contrast studies. Br J Radiol 1980;53:576–9. [DOI] [PubMed] [Google Scholar]
- [27].McCollough CH, Primak AN, Braun N, et al. Strategies for reducing radiation dose in CT. Radiol Clin North Am 2009;47:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shin CI, Kim SH, Lee ES, et al. Ultra-low peak voltage CT colonography: effect of iterative reconstruction algorithms on performance of radiologists who use anthropomorphic colonic phantoms. Radiology 2014;273:759–71. [DOI] [PubMed] [Google Scholar]
- [29].Huda W, Scalzetti EM, Levin G. Technique factors and image quality as functions of patient weight at abdominal CT. Radiology 2000;217:430–5. [DOI] [PubMed] [Google Scholar]


