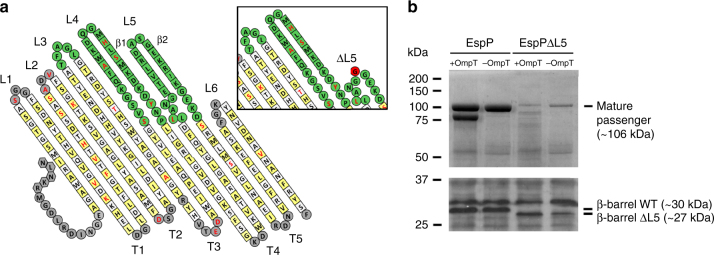
Fig. 3.
Truncation of L5 affects folding of the EspP passenger domain. a Left panel: Topology model of the EspP β-barrel domain based on its crystal structure (PDB code 3SLJ). Yellow and white squares indicate residues with side chains that point toward the lipid bilayer and the β-barrel lumen, respectively. Residues in periplasmic turns labeled T1-T5 and extracellular loops labeled L1, L2, and L6 are shown in gray, as are the residues in the α-helical segment. Residues in extracellular loops labeled L3, L4, and L5 are shown in green and residues that differ in Pet are highlighted in red font. Right panel: topology model of the EspP β-barrel domain showing the L5 truncation created by replacing E1238–K1251 with one glycine residue (shown in red). b SDS-PAGE of TCA-precipitated culture supernatant fractions (top panel) and immunoblotting of whole-cell lysates with anti-Pet β-barrel domain antibodies* (bottom panel) collected after growth of E. coli TOP10 ( + OmpT) and BW25113ΔompT (− OmpT) expressing EspP and EspPΔL5. Image is representative of at least two independent experiments. *The Pet and EspP β-barrel domains are so similar that anti-Pet β-barrel domain antibodies cross-react with the EspP β-barrel domain