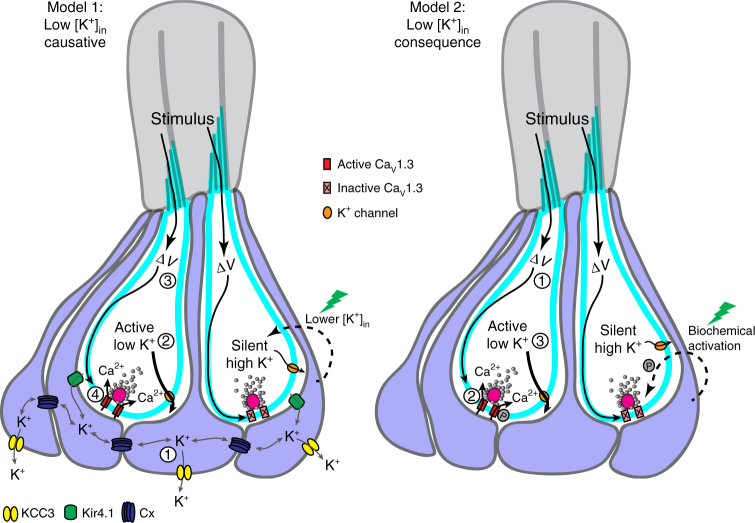
Fig. 8.
Model of presynaptic silencing in neuromast organs. Left-side, Model 1: low [K+]in is causative for CaV1.3 channel (red) activation. In active cells, (1) similar to glia, supporting cells may use Kir4.1 channels (green) to take up [K+]ex, gap junctions (Cx, blue) to spatially buffer K+ among syncytia of supporting cells, and a K+-Cl− cotransporter such as KCC3 (yellow) to clear K+ from supporting cells. This results in (2) lower [K+]in in active cells. Cells with lower [K+]in may be at sufficiently depolarized resting membrane potentials where (3) mechanosensation and depolarization (∆V) is able to (4) activate CaV1.3 channels. In this model, despite K+ buffering by supporting cells, not all hair cells are able to be maintained with [K+]in levels low enough to facilitate presynaptic function. Blocking gap junctions elevates hair-cell [K+]in and presynaptic function is lost in all cells. After laser damage (green lightning bolt), a signaling cascade could lower [K+]in levels in silent cells, and raise resting membrane potentials into a range suitable for CaV1.3 channel activation. Right-side, Model 2: low [K+]in is a consequence of presynaptic activity. This schematic demonstrates that (1) mechanosensation and depolarization (∆V), lead to (2) CaV1.3 channel activation in active cells. Presynaptic activity results in (3) lower [K+]in. In this model, CaV1.3 channels may be inactive due to a lack of biochemical modification, such as phosphorylation (P), as shown in this example. Alternatively, CaV1.3 channels could also be rendered inactive due to a missing interaction partner or improper assembly at the plasma membrane (these examples not shown). In this example, after laser damage (green lightning bolt), a signaling cascade could promote phosphorylation and lead to CaV1.3 channel activation