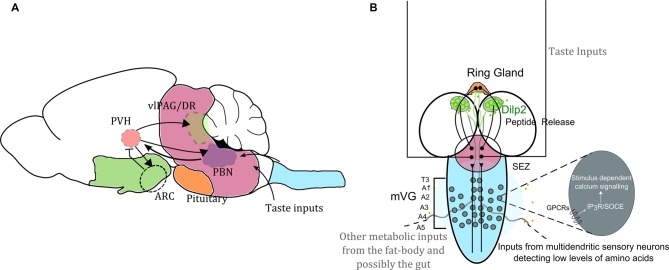
Figure 1.
Neuronal circuit motifs regulating feeding and satiety are conserved between mammals and Drosophila. Comparison of circuits regulating feeding and satiety in mammals (A) and Drosophila larvae (B) equates the hypothalamus with the medial neurosecretory cells (mNSC; green), the pituitary region to the ring gland (orange), the brain stem to the sub-oesophageal ganglion (SOG or the sub-oesophageal zone, SEZ; pink) and the spinal cord to the ventral ganglion (blue). Studies have shown that the agouti-related peptide (AgRP) neurons and pro-opiomelanocortin (POMC) neurons housed in the arcuate nucleus (ARC) are critical for driving feeding behavior. However, in the absence of these two key cell types, feeding and satiety are also influenced by the paraventricular nucleus (PVH of the hypothalamus), which activates the cells in the ARC as well as drives activity in the para-brachial nucleus (PBN) for attaining satiety (Wu et al., 2009, 2012; Sternson and Eiselt, 2017). Glutamatergic interneurons in the mid-ventral ganglion (mVG) similarly activate the mNSC to regulate response to starvation (Jayakumar et al., 2016). In (A) the arrowheads show activation, and the arrow with the flatline indicates inhibition. The various regions are important for achieving satiety. In (B) we also highlight that intracellular calcium signaling mechanisms through various G-protein coupled receptors (GPCRs) involving both the inositol-1,4,5-trisphosphate receptor (IP3R) mediated calcium release as well as store-operated calcium entry (SOCE) in the mVG neurons possibly allow integration of environmental dietary quality with internal metabolic state. Hence the with internal metabolic state. Hence the mVG-mNSC connection in Drosophila larvae may be a precursor of an evolved and complex spinal.