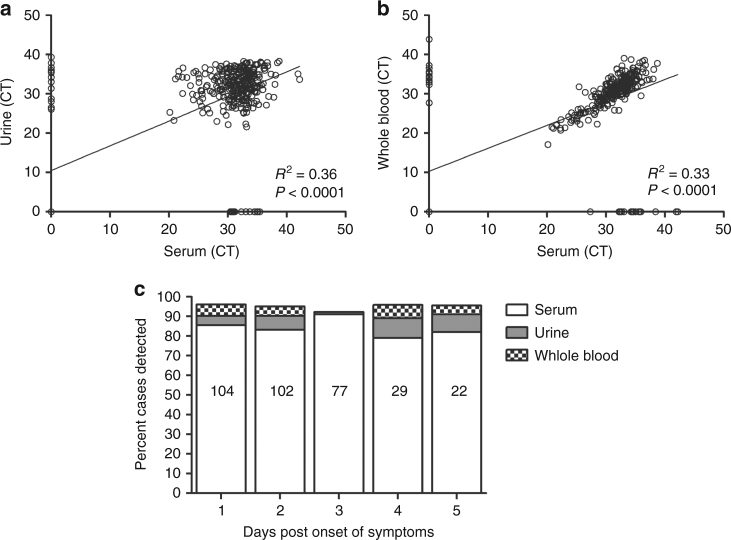
Fig. 5.
Clinical performance of the Trioplex assay across specimen types. Clinical specimens collected concurrently from 373 cases with previous Zika determination in the acute stage were tested. RNA was extracted with the MagNA Pure 96 small volume external lysis protocol from 373 case-paired serum, 373 urine, and 345 whole blood-EDTA specimens and tested with the Trioplex assay in multiplex format in the ABI 7500 Fast Dx instrument. a Correlation of CT values between case-matching serum and urine specimens; R2 = 0.36 p < 0.0001. b Correlation of CT values between case-matching serum and whole blood-EDTA specimens; R2 = 0.33 p < 0.0001. c Dataset separated by DPO 1–5 and percent cases detected by the Trioplex assay in every specimen type. The white bar represents the percent of cases with ZIKV detected in serum specimens. The gray bar represents the percent of ZIKV-positive urine specimens that resulted ZIKV-negative in the matching serum specimen, and the checkered box represents the percent of ZIKV-positive whole blood specimens resulted ZIKV-negative in the matching serum specimen. Both gray and checkered boxes represent the added value of testing more than one specimen type with the Trioplex assay