Abstract
Background
Scarlet fever is caused by a group A streptococcal (GAS) infection. On April 3, 2017, an outbreak among children in a kindergarten was reported to the local health department. An epidemiologic investigation was conducted to identify the possible transmission route of this outbreak and to recommend appropriate control measures.
Materials and Methods
A retrospective cohort study was conducted using questionnaires including age, sex, the classroom attended at a kindergarten, and date and type of symptoms developed. A case-patient is defined as a child having sore throat, fever, skin rash, or strawberry tongue with or without laboratory confirmation of GAS infection between March 28 and April 28, 2017.
Results
The index case-patients developed symptoms on March 28, 2017, and this outbreak persisted over a period of 16 days. The outbreak affected 21 out of 158 children (13.3%) in the kindergarten, with the mean age of 4.2 (range 3–5) years; 12 (57.1%) of them were boys. The common symptoms reported were fever (71.4%), sore throat (71.4%), reddened tonsil (57.1%), and skin rash (52.4%). The epidemiologic analysis showed that children attending one of the classrooms in the kindergarten were 14.12 times affected than the other classrooms (relative risk, 14.12; 95% confidence interval, 4.99–33.93; P <0.01). All case-patients were recommended to stay away from the kindergarten and its social activities for >24 hours after starting appropriate antibiotic treatment, and all the children in the kindergarten were instructed to keep strict personal hygiene practices.
Conclusion
Our results suggest that the outbreak likely affected from the index case-patients who attended to one of the classrooms in the kindergarten. This highlights the importance of immediate notification of outbreak to prevent large number of patients.
Keywords: Scarlet fever, Outbreak, Children, Kindergarten, Korea
Introduction
Scarlet fever is an infection caused by group A streptococcus (GAS), Streptococcus pyogenes, which produces a pyrogenic exotoxin [1]. GAS causes diverse clinical symptoms associated with sudden onset of fever (38.5°C), sore throat, strawberry tongue, and skin rash [2]. The incubation period ranges from 2 to 10 days and the transmission usually persist from 10 to 21 days without appropriate treatment [2,3]. Antibiotic (penicillin) administration for 10 days is the treatment of choice with the loss of transmissibility within a day following proper antibiotic treatment [2]. Scarlet fever was known to be highly fatal in the early 1900s; however, it only causes mild illness recently due to the development of antibiotics and improvement of hygiene and living environment [3]. Nevertheless, the annual number of scarlet fever reported has greatly increased from 0.3 cases/100,000 persons in 2008 to 23.08 cases/100,000 persons in 2016 in the Republic of Korea, and 8.98 cases/100,000 persons in 2013 to 27.77/100,000 persons in 2016 at Gyeonggi Province, Korea [4].
On April 3, 2017, an outbreak of scarlet fever among children at a kindergarten was reported to the local public health department by the local pediatric clinic. A field epidemiologist from Gyeonggi Provincial Government conducted an investigation and implemented control measures.
Here, an epidemiological analysis was conducted to identify the possible route of transmission and to recommend appropriate control measures to prevent future outbreaks of scarlet fever.
Materials and Methods
The need for ethical approval for this study was waived, based on the Korean Infectious Disease Control and Prevention Act. No. 4 and Enforcement Rule of Bioethics and Safety Act. No. 33.
1. Case definition
Scarlet fever has been designated as a notifiable disease with a case defined by the Korean Centers for Disease Control and Prevention [5]. Probable case is defined as the person who has symptoms such as sore throat, fever, skin rash, or strawberry tongue without laboratory confirmation of GAS infection. Confirmed case is defined as the person with identified GAS in his or her pharynx or blood among probable cases.
Carriers are those who have GAS in their pharynx without presenting any symptoms during the study period. All cases were based on the notification from the physician and staff in a kindergarten during the period between March 28 and April 28, 2017.
2. Study design
A retrospective cohort study was conducted to identify the possible transmission route. A total of 19 confirmed and 2 probable cases were enrolled as a case group. Eight carriers and 129 children without infection were enrolled as a non-case group. Data including age, sex, the classroom attended at a kindergarten, underlying disease, date and type of symptoms developed, and presence of illness from their household were collected during the investigation.
3. Carriage study
A screening was performed in the kindergarten on April 4, 2017. Forty-four children (diagnosed cases, 21; refusal, 6; absence, 17) were excluded from the screening test among the 158 children and 9 staff. The provincial field epidemiologist and a physician from the city public health department performed the screening by swabbing the posterior pharynx and tonsils.
4. Detection of GAS
Rapid antigen GAS tests were performed using an immunochromatographic method for 14 swab samples at the local clinics during the diagnosis (BD Veritor System for Rapid Detection of GAS, BD Rapid Diagnostics Co. Ltd, Suzhou, China). Five swab samples collected from the local clinics and 123 specimens collected for screening carriers from the kindergarten were sent to the provincial public health laboratory for GAS identification using the Vitek II system (bioMerieux, Marcy l'Etoile, France).
5. Statistical analysis
Overall attack rate was calculated using the number of cases divided by the total number of children in the kindergarten. Secondary attack rate was calculated using the number of cases divided by the total number of siblings. To evaluate the risk factor on the route of transmission, relative risks (RRs), with the corresponding 95% confidence intervals (CIs), and Fisher's exact test were calculated. A P-value of <0.05 was considered statistically significant. The statistical package R version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
Results
1. Description of the kindergarten
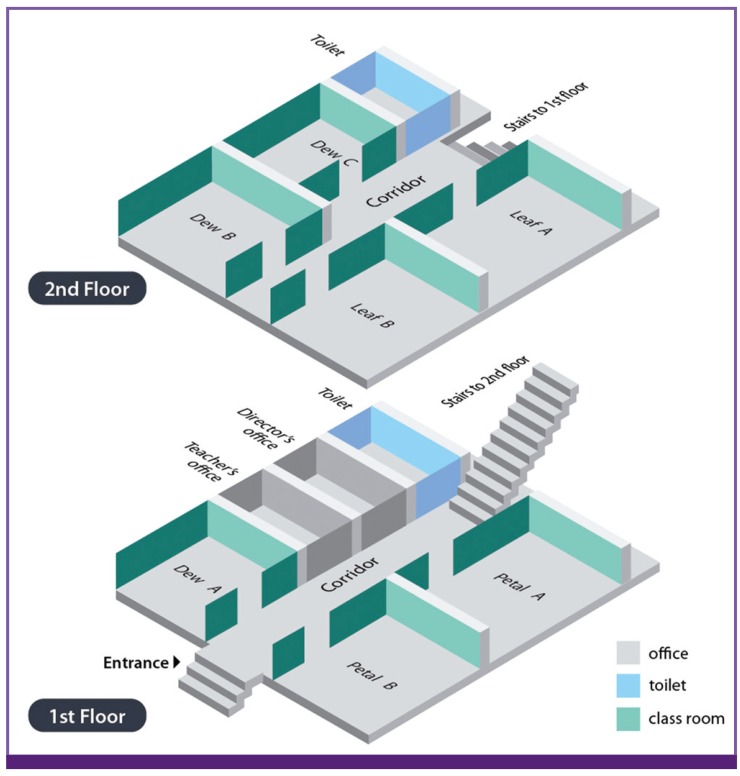
The kindergarten has seven classrooms with two floors. The first floor includes classrooms named Petal A, Petal B, and Dew A, and second floor includes Leaf A, Leaf B, Dew B, and Dew C (Figure 1). Each floor has a toilet and was connected with a stair. During investigation, a total of 158 children and 9 teachers were present. No large-sized group activity among classrooms was found in the recent months, and meals were provided in each classroom.
Figure 1.
Floor plan of the kindergarten with the name of classroom
2. Descriptive epidemiology
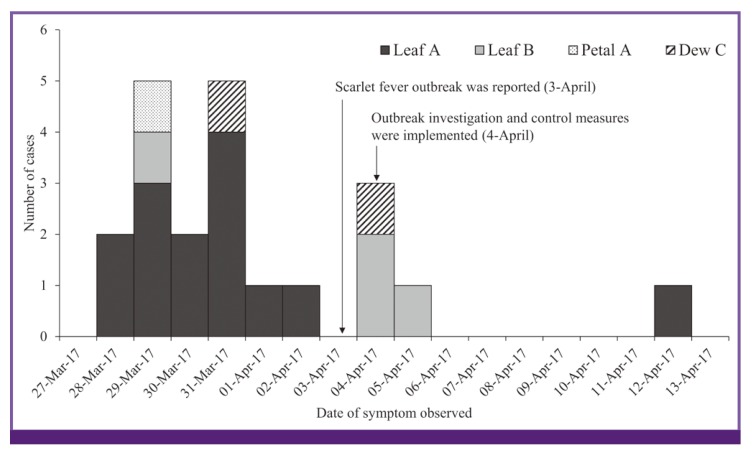
The outbreak affected 21 out of 158 children in the kindergarten with 19 confirmed and 2 probable cases: 12 (57.1%) boys and 9 (42.9%) girls. The median age was 4.2 ranging from 3 to 5 years. Antibiotics (amoxicillin) were introduced to all cases from the local primary care clinic without hospitalization, and complication of infection was not identified. The index cases, attendees at the same class (named Leaf A), had symptoms such as fever, sore throat, and skin rash since March 28, 2017, and were quarantined and administered with antibiotics the day after the symptom developed according to the physician's recommendation. This outbreak was reported on April 3, 2017 and persisted over a period of 16 days (Figure 2). The most common symptoms reported were sudden onset of fever and sore throat (15 cases, 71.4%). Reddened tonsil and scarlatinoid rash were reported in 12 (57.1%) and 11 (52.4%) cases, respectively. Three cases (14.3%) had headache and vomiting, and two cases (9.6%) had strawberry tongue. Two household transmissions were found from two cases, which started on March 28 and March 29, 2017. They were all siblings of the patients, and appropriate antibiotic treatment with quarantine was administered.
Figure 2.
Epidemic curve by the date of symptom onset with the name of classroom
3. Analytical epidemiology
The overall attack rate was 13.3%; attack rates based on the kindergarten class were 45.1% in Leaf A, 14.3% in Leaf B, 13.3% in Dew C, and 4.2% in Petal A (Table 1). The RR of the children attending class Leaf A was 14.12 (95% CI, 4.99–33.93, P<0.01), whereas those of other classrooms were not statistically significant. A total of two cases had secondary infection among the total five siblings, with a secondary attack rate of 40%.
Table 1. Attack rate and relative risk of each classroom in the kindergarten associated with case development.
| Classroom | Attendee | Non-attendee | Relative risk | 95% CI | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Case | AR (%) | Total | Case | AR (%) | ||||
| Leaf A | 31 | 14 | 45.1 | 127 | 7 | 5.5 | 14.12 | 4.99–33.93 | <0.01 |
| Leaf B | 28 | 4 | 14.3 | 130 | 17 | 13 | 1.11 | 0.34–3.59 | 0.86 |
| Dew C | 15 | 2 | 13.3 | 143 | 19 | 13.3 | 1.00 | 0.21–4.80 | 0.99 |
| Petal A | 24 | 1 | 4.2 | 134 | 20 | 14.9 | 0.25 | 0.03–1.94 | 0.15 |
CI, confidence interval; AR, attack rate.
4. Carriage studies
GAS was not isolated from the staffs in the kindergarten. However, it colonized in six (5.3%) of 114 children without showing any abnormal symptoms. Among them, four were on the first floor and two were on the second floor, which were not significantly different.
5. Control measures
On April 4, 2017, the first day of investigation, cases were recommended to stay away from the kindergarten and social activities for 24 hours after starting appropriate antibiotic treatment. Furthermore, environmental disinfection in the bookshelves, doorknobs, and toys in all classrooms in the kindergarten was performed, and personal hygiene education to kindergarteners including hand washing was conducted. In addition, to immediately identify the cases, daily health check-up was conducted by the staffs of the kindergarten by asking kindergarteners any abnormal symptoms such as sore throat and fever until April 30, 2017.
DISCUSSION
The results suggest that this infection likely originated in the children who attended class Leaf A and then spread to other nearby classrooms. The reasons are the following: (1) GAS had been identified from the cases whose symptoms started during the first day of the outbreak; (2) children in class Leaf A were at high risk of getting infection (RR, 14.12; P <0.01) than the other classrooms; and (3) the epidemic curve showed the high incidence during a relatively short period of time, suggesting the cases in the early phase of this outbreak might have been exposed to the same index case and the transmission propagated later on.
Person-to-person transmission with respiratory droplet or direct contact with other persons is the most common route of GAS transmission [2]. A previous study supports our results that the duration of exposure and the distance from the index case are the major risk factors of GAS transmission [6]. Furthermore, two siblings of the cases developed the symptoms on the first and second day of this outbreak.
GAS can be environmentally transmitted, such as in the surface of the furniture and the floor [7]. Thus, environmental cleaning with disinfectants such as alcohol or chlorine was included as a control measure for this outbreak.
The staff's delayed identification and notification of this outbreak were considered as the major causes of the large number of cases, which can be avoided by immediately reporting the occurrence of an outbreak and implementing appropriate control measures.
The carriers in this study were not treated with antibiotics, because of limited evidence of infection and treatment effectiveness [8]. Furthermore, a previous study showed that 3–17% of children younger than 5 years are asymptomatic carriers of GAS in their pharynx, which is consistent with our results (5.3%) [9].
Several studies have reported an increasing rate of scarlet fever in countries including the Republic of Korea in the recent years, prompting increased attention to the epidemiological factors that caused the spread of GAS particularly in young children [10,11]. The reasons behind this increase in Korea are unclear, but may be attributable to the extending criteria of the national notification system by including probable cases since 2011 [10]. Other literatures demonstrated that natural fluctuation of immunity and antimicrobial resistance are considered as factors affecting the recent increase [12,13]. thus, previous studies suggest that, monitoring GAS strain with antibiotic susceptibility is critical to prevent future outbreaks of scarlet fever [10,14].
This study has several limitations. First, the infection source was not clearly identified, because the index case could not remember the place visited and person contacted. Second, the specific type of gene sequence of GAS that caused this outbreak among cases was not identified. However, based on the epidemiologic link with consecutive timeline among cases and no other cases reported in the region during the outbreak, the infection was most likely caused by the same pathogen. Third, rapid antigen streptococcus test that was used to identify cases may not be sensitive enough to detect GAS infection [15]. However, the clinical symptoms of all cases are highly consistent with GAS infection.
Acknowledgements
The authors would like to thank the colleagues at the Sungnam city and provincial's department of public health for their assistance with the investigation and the microbiologists from the provincial public health laboratory for immediately providing us with the laboratory test results.
Footnotes
Conflicts of Interest: No conflicts of interest.
References
- 1.Wong Samson SY, Yuen KY. Streptococcus pyogenes and re-emergence of scarlet fever as a public health problem. Emerg Microbes Infect. 2012;1:e2. doi: 10.1038/emi.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heymann DL. Control of communicable disease manual. 19th ed. Washington, DC: American Public Health Association; 2008. pp. 577–585. [Google Scholar]
- 3.Lamden KH. An outbreak of scarlet fever in a primary school. Arch Dis Child. 2011;96:394–397. doi: 10.1136/adc.2010.189944. [DOI] [PubMed] [Google Scholar]
- 4.Korea Centers for Disease Control and Prevention (KCDC) Infectious disease statistics system. [Accessed 21 September 2017]. Available at: https://is.cdc.go.kr/dstat/index.jsp.
- 5.Korea Center for Disease Control and Prevention (KCDC) Case definition for national notifiable infectious disease. Osong: KCDC; 2016. [Google Scholar]
- 6.Weiss K, Laverdière M, Lovgren M, Delorme J, Poirier L, Béliveau C, Group A. Streptococcus carriage among close contacts of patients with invasive infection. Am J Epidemiol. 1999;149:863–868. doi: 10.1093/oxfordjournals.aje.a009902. [DOI] [PubMed] [Google Scholar]
- 7.Wagenvoort JH. 1, Penders RJ, Davies BI, Lütticken R. Similar environmental survival patterns of Streptococcus pyogenes strains of different epidemiologic backgrounds and clinical severity. Eur J Clin Microbiol Infect Dis. 2005;24:65–67. doi: 10.1007/s10096-004-1256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan EL. Group A streptococcal carriers and contacts: (when) is retreatment necessary? In: Shulman S, editor. Management of pharyngitis in an era of declining rheumatic fever. Columbus, OH: Ross Conference on Pediatric Research; 1984. p. 92. [Google Scholar]
- 9.Shaikh N, Leonard E, Martin JM. Prevalence of Streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010;126:e557–e564. doi: 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- 10.Park DW, Kim SH, Park JW, Kim MJ, Cho SJ, Park HJ, Jung SH, Seo MH, Lee YS, Kim BH, Min H, Lee SY, Ha DR, Kim ES, Hong Y, Chung JK. Incidence and characteristics of scarlet fever, South Korea, 2008-2015. Emerg Infect Dis. 2017;23:658–661. doi: 10.3201/eid2304.160773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner CE, Pyzio M, Song B, Lamagni T, Meltzer M, Chow JY, Efstratiou A, Curtis S, Sriskandan S. Scarlet fever upsurge in England and molecular-genetic analysis in North West London, 2014. Emerg Infect Dis. 2016;22:1075–1078. doi: 10.3201/eid2206.151726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan JJ, Liu CC, Ko WC, Hsu SY, Wu HM, Lin YS, Lin MT, Chuang WJ, Wu JJ. Molecular analysis of group A streptococcal isolates associated with scarlet fever in southern Taiwan between 1993 and 2002. J Clin Microbiol. 2003;41:4858–4861. doi: 10.1128/JCM.41.10.4858-4861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tse H, Bao JY, Davies MR, Maamary P, Tsoi HW, Tong AH, Ho TC, Lin CH, Gillen CM, Barnett TC, Chen JH, Lee M, Yam WC, Wong CK, Ong CL, Chan YW, Wu CW, Ng T, Lim WW, Tsang TH, Tse CW, Dougan G, Walker MJ, Lok S, Yuen KY. Molecular characterization of the 2011 Hong Kong scarlet fever outbreak. J Infect Dis. 2012;206:341–351. doi: 10.1093/infdis/jis362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy R, Williams C, Irvine N, Reynolds A, Coelho J, Saliba V, Thomas D, Doherty L, Chalker V, von Wissmann B, Chand M, Efstratiou A, Ramsay M, Lamagni T. Increase in scarlet fever notifications in the United Kingdom, 2013/2014. Euro Surveill. 2014;19:20749. doi: 10.2807/1560-7917.es2014.19.12.20749. [DOI] [PubMed] [Google Scholar]
- 15.Stewart EH, Davis B, Clemans-Taylor BL, Littenberg B, Estrada CA, Centor RM. Rapid antigen Group A streptococcus test to diagnose pharyngitis: a systematic review and meta-analysis. PLoS One. 2014;9:e111727. doi: 10.1371/journal.pone.0111727. [DOI] [PMC free article] [PubMed] [Google Scholar]