Figure 2.
Intracellular Calcium Responses and Electrophysiology Reveals Functional NMDAR in βTC-B6 but Not in βTC-C3H Cells
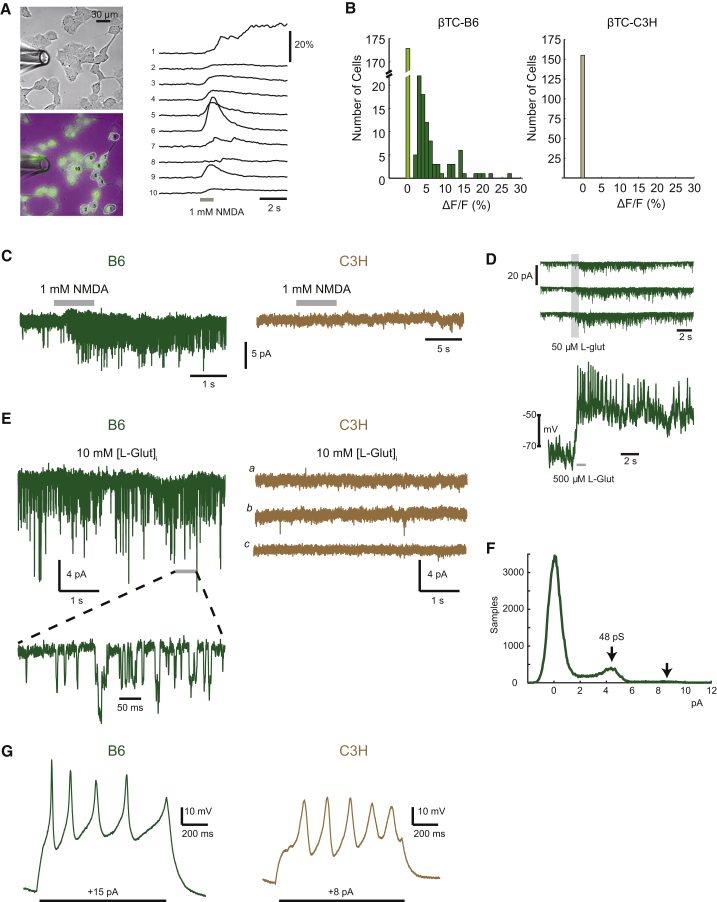
(A) Oregon Green-labeled calcium indicator BAPTA-AM was applied to βTC-B6 and βTC-C3H cancer cells bathed in a Mg-free Ringer solution; puffing an NMDA solution (1 mM, 1 s, through perfusion pipette at left) induced calcium influx into the cells, thereby producing an increased fluorescence signal (ΔF) compared with the background fluorescence signal (F). The top left image shows βTC-B6 in phase-contrast, whereas the lower left image shows a green-fluorescence signal overlaid with a phase-contrast image. The graph at the right shows time-resolved fluorescence signals (sampling frequency/frame rate = 12.5 Hz), where each trace represents one recorded cell. The y axis indicates the change in fluorescence intensity.
(B) Using the fluorescence reporter assay in (A), the number of βTC cells with active NMDAR signaling was determined following puff application of 1 mM NMDA. The ΔF/F measurements refer to the normalized difference in each cell's signal measured immediately before the application of agonist compared with the peak of the response after the puff. For βTC-B6, 263 cells from 15 different regions of three independent culture dishes were recorded. Light green bar indicates cells with no response; dark green bars indicate cells with ΔF. For βTC-C3H, 155 cells from eight regions of two different dishes were analyzed. Wilcoxon rank-sum test, p < 4.24e−16.
(C) Left: exogenous application of 1 mM NMDA to βTC-B6 cells, using a puffer pipette pressure application during the period shown by gray bar. (Low-noise whole-cell recording, holding at −90 mV.) Right: exogenous application of 500 μM NMDA (two cells), 1 mM NMDA (seven cells), or glutamate (50 μM, four cells) to βTC-C3H cells.
(D) L-Glutamate application to βTC-B6 cancer cells. Three successive membrane current responses (in voltage-clamp mode) are shown, using Mg-free Ringer solution, with a membrane potential of −80 mV (upper panel); voltage-response, including action potentials, was measured in current-clamp (i.e., voltage recording) mode (lower panel).
(E) Intracellular glutamate perfusion during low-noise whole-cell recordings to assess autocrine activation of NMDARs in βTC cells. Left: βTC-B6 cells, n = 12 cells. A segment at higher time resolution is shown at bottom, as indicated. Right: βTC-C3H cells, n = 9 cells. (a–c) Representative segments of recording from one of three different cells.
(F) Current amplitude histogram of autocrine-activated NMDARs in a βTC-B6 cell, showing peaks corresponding with single and double openings of channels (indicated by arrows) with a chord conductance of 48 pS, assuming reversal at 0 mV.
(G) Current-clamp recordings with step current injection in βTC-B6 (left) and βTC-C3H (right).