Table 2.
Scope of the Enantioselective α-Benzylation of Aldehydes with Alcoholsa
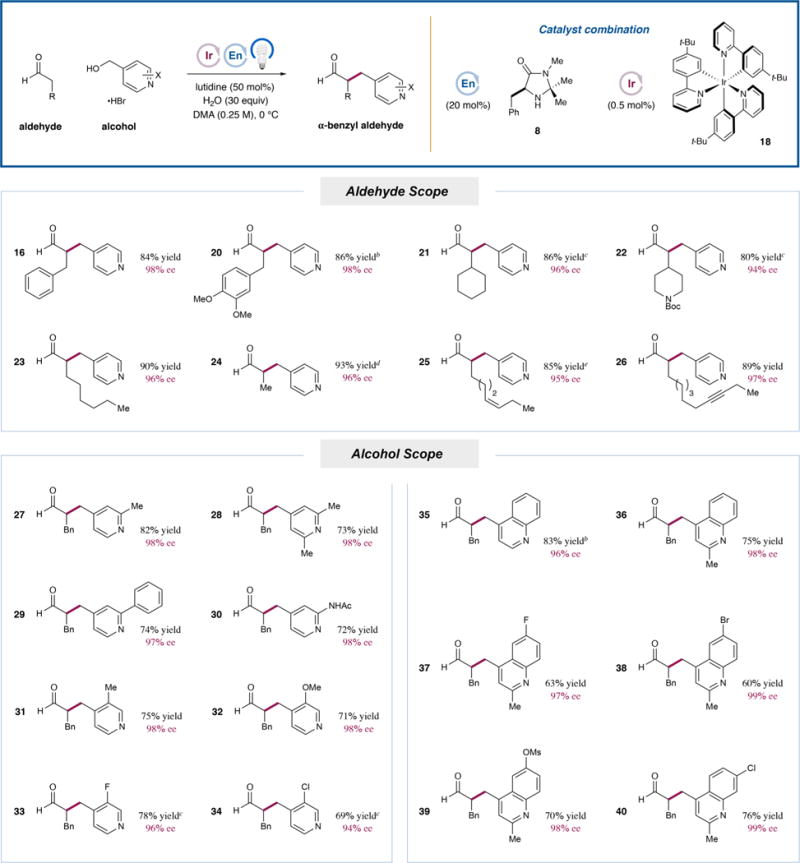
|
Alcohol (0.5 mmol), aldehyde (2.0 equiv), lutidine (50 mol %), water (30 equiv), organocatalyst 8 (20 mol %), and photocatalyst 18 (0.5 mol %) were irradiated in DMA with a 34 W blue LED lamp at 0 °C. Isolated yields are reported. Enantioselectivities were determined by chiral HPLC analysis following reduction of the crude aldehydes to the corresponding alcohols.
Characterized as the corresponding alcohol.
Aldehyde (5.0 equiv).
Yield determined by 1H NMR.
From the Z-starting material, 25 was obtained as a 4.5:1 mixture of Z and E isomers; chiral HPLC analysis was performed following reduction of the crude aldehyde to the corresponding alcohol and subsequent hydrogenation of the alkene.
