Table 4.
Leaving Group Scope in the Enantioselective Spin-Center Shift-Enabled α-Benzylation of Aldehydes and Its Impact on Reactivitya
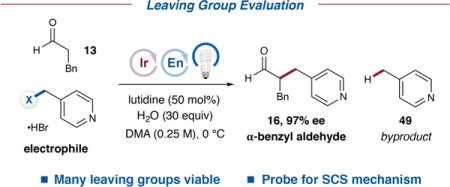
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| entry | X | Ered (V) | Ksv(mM−1) | pKa (XH) | yield (2 h) | yield [time] | 16:49 |
| 1 | OAc | −1.19 | 0.84 | 4.76 | 75% | 90% [3 h] | 18 |
| 2 | NMe3+Br− | n.d.b | 0.13 | 9.80 | 67% | 86% [5 h] | 14 |
| 3 | OH | −1.29 | 1.05 | 15.7 | 39% | 85% [5 h] | 7.7 |
| 4 | OMe | −1.29 | 1.15 | 15.2 | 29% | 71% [24 h] | 5.1 |
| 5 | OTBDPS | −1.30 | 0.82 | ⪝13.6 | 20% | 61% [48 h] | 3.1 |
|
| |||||||
| 6 | – | −1.30 | 1.30 | data for pyridine·HBr | |||
Electrophile (0.25 mmol), aldehyde 13 (2.0 equiv), lutidine (50 mol %), water (30 equiv), organocatalyst 8 (20 mol %), and photocatalyst 18 (0.5 mol %) were irradiated in DMA with a 34 W blue LED lamp. Yields of 16 and 49 were determined by 1H NMR. Enantioselectivities were determined by chiral HPLC analysis following reduction of the crude aldehyde to the corresponding alcohol. Acidity data in water from ref 24. See the SI for full experimental details.
Low solubility prevented electrochemical measurements in aprotic solvents.
