Abstract
Cochrane is a global organization committed to carrying out high-standard systematic reviews and meta-analyses to inform health care and those associated with it, from patients to providers. The Cochrane Tobacco Addiction Group (TAG) has been reviewing the evidence for interventions to treat and prevent tobacco addiction for 20 years. During this time, the group has published over 70 reviews in the area, which have had substantial impacts on health care guidance and treatment provision. This has coincided with a reduction in smoking prevalence in the United Kingdom. One of the groups’ key objectives is to move with the times, and it does this not only by updating historical reviews with the most up-to-date evidence but also by commissioning or accepting requests for new reviews in novel areas, such as electronic cigarettes and plain packaging. This review paper highlights the previous important work that the group has done and its impacts, what is happening within the group more currently, and also describes where Cochrane TAG wish to go in the future and the work being done to solidify aims. Part of this is a prioritization project being carried out to mark the 20th anniversary of the group, which is using stakeholder engagement to inform an action plan to inform the outputs and aid dissemination to ensure Cochrane TAG’s work is relevant and maximizes impact for the next 20 years.
Implications
This review provides an overview of the work of Cochrane TAG. Readers will gain an insight into the origins of the group, its impact on evidence-based medicine relating to tobacco addiction, and the goals of the group moving forward. This supports the group’s aim to encourage knowledge of Cochrane’s work within the field, and thereby the wider use of and contribution to high-quality systematic reviews and meta-analyses of the literature to improve policy and clinical practice.
Introduction
Tobacco is the leading cause of preventable death worldwide, and the health impacts associated with use are substantial and diverse. Tobacco accounts for 80% of deaths due to lung cancer; 80% of deaths due to bronchitis and emphysema; and 14% of deaths related to ischemic heart disease. It also causes over 25% of cancers including bladder, lip, throat, mouth, kidney, pancreas, and cervix.1 On average, smokers die 10 years before their nonsmoking counterparts.1 In order to reduce such morbidity and mortality, the establishment of effective evidence-based policies has become a major global health priority. Due to the vast amount of material available on the subject, however, establishing which interventions are most effective is often difficult for policy makers.
It has been estimated that the total cost to the United Kingdom of tobacco smoking is £13.9 billion per year,2 and therefore, returns on investments into tobacco control are likely to be great. The UK All Party Parliamentary Group on Smoking and Health recently estimated that increasing the UK government expenditure by £100 million per year would likely see a 1100% return on the investment over 5 years.2 Providing a reliable, evidence-based framework will enable investments to be used wisely and potential health and economic returns maximized (see Box 1 for a very brief history of evidence-based medicine).
Box 1. History of Evidence-Based Medicine
“Evidence-based medicine” was a phrase first used by Gordon Guyatt, an internal medicine resident at Mcmanus University in the early 1990s.3 He had benefited from mentoring by Dr David Sackett, a clinician with public health training, who was responsible for the implementation of the first clinical epidemiology course at McManus University in 1967 and was the first to describe “critical appraisal.”4
Others too had sought to increase the scientific rigor of clinical decision making. Dr Alvan Feinstein used his mathematical background to collect data about patients from the Rheumatic Fever Hospital in New York. He knew that there were biases and errors in interpreting murmurs at the bedside and used his epidemiological data to transform the care these patients received, ultimately resulting in the closure of the hospital due to lack of patients.5 He was also the first to use the phrase “clinical epidemiology”6–8 in three papers published in the Annals of Internal Medicine.
Suzanne and Robert Fletcher were also substantial contributors to the formation of this new movement. Both doctors had recognized the gap between research and clinical decision making and after qualifying in clinical medicine and public health established a medical epidemiology course at McGill University Medical School and published Clinical Epidemiology: The Essentials in 1982. Prior to this, the accepted way to practise medicine was predominantly expert opinion, and there was little translation of epidemiology or trial data into clinical decision making.
Since 1996, the University of Oxford’s Cochrane Tobacco Addiction Group (TAG) has systematically collated and reviewed studies relating to tobacco interventions. These evidence-based reviews have been used to inform changes in policy and clinical practice guidelines for tobacco use globally. The aim of this paper is to give a background to the origins of the group, highlight some of the important work carried out to date and its impact, review some recent findings, and look to the future by identifying some upcoming projects.
Cochrane TAG
Cochrane TAG currently have a suite of approximately 70 completed reviews on the topic of tobacco use treatment and prevention, with many including meta-analyses of primary study data. They also manage some “orphan” reviews—systematic reviews on topics that do not fit within any other subject-specific Cochrane review groups—on subjects from eLearning in health care to food allergies. The group has a team made up of hundreds of external review authors who they assist to carry out reviews; however, TAG’s editors also author a number of reviews themselves (see Box 2 for a brief history of Cochrane and Box 3 for a history of Cochrane TAG).
Box 2. What Is Cochrane?
The formation of Cochrane can be attributed to three individuals: Iain Chalmers, Tom Chalmers, and Murray Enkin. The organization was named after Archie Cochrane, who was the first individual to promote the use of randomized controlled trials (RCTs) to reduce study biases. As a prisoner of war in World War 2, he used randomization on his fellow prisoners to study whether yeast extract affected the development of deficiency disease.9 Later, he was known for the Rhodda Fach study,10 which looked at the role of tuberculosis versus dust in the development of progressive pulmonary fibrosis. Tom Chalmers recognized the importance of Archie Cochrane’s work and was the first to describe a hierarchy of evidence, culminating in RCTs, systematic reviews, and meta-analyses and to highlight the risks of publication bias.11 Tom Chalmers and Iain Chalmers collaborated with obstetrician Murray Enkin to pool all the current study data relating to the care of pregnant women, which ultimately led to the publication of “Effective Care in Pregnancy and Childbirth”12 and the formation of the Cochrane Collaboration in 1993. Early results from the group were instrumental in revolutionizing care in the perinatal period, including the final cessation of the use of diethylstilbestrol and role of administering steroids to mothers of preterm infants.
Today, Cochrane (as it is now more simply called) is a global not-for-profit organization dedicated to reviewing the available health care literature, with a strong emphasis on detecting potential biases. It does this using systematic review and meta-analyses methods, which are detailed in the Cochrane Handbook. The aim of this is to provide a high-quality evidence base to inform health care decisions. It is made up of over 50 topic-specific review groups who review the literature in particular topic areas.
Box 3. History of the Cochrane Tobacco Addiction Group
Cochrane is an organization made up of many Review Groups (see Box 2). Cochrane Cochrane Tobacco Addiction Group (TAG) was one of the first Cochrane Review Groups to be established. It was founded by Chris Silagy, Tim Lancaster, and Godfrey Fowler, who were all general practitioners working in the General Practice Research Group (GPRG), within the Department of Public Health, University of Oxford. GPRG had a research focus on smoking cessation and had conducted one of the first trials of the use of nicotine patches for smoking cessation in a primary care setting.
Through his work in Oxford, Chris Silagy had met Iain Chalmers, who was working on establishing the Cochrane Collaboration, and Chris became involved in helping to drive the concept forward. The first review produced by TAG was a systematic review of nicotine replacement therapy (NRT) for smoking cessation. This was a prototype Cochrane review used to test the methodologies and software of the collaboration. This review, many times updated, remains in the Cochrane Library 24 years later. Work to go on and review other smoking cessation interventions was initially funded by the Imperial Cancer Research Fund (ICRF)—now known as Cancer Research UK (CRUK). In 1995, the decision was made to establish a Cochrane Review Group focusing on interventions to prevent uptake of smoking and to help people to quit. From 1996, this work was funded by the NHS Research and Development stream and later by the National Institute for Health Research, who still fund the group today.
Following the NRT review, the next TAG reviews focused on physician advice,13 acupuncture and hypnotherapy to help people quit smoking,14 followed by a review covering anxiolytic and antidepressant pharmacotherapies.15
The aims of Cochrane TAG’s research are:
to inform tobacco control policy internationally;
to inform research in tobacco control and to help ensure new research is focused on important unanswered questions; and
to contribute to reducing tobacco use.
Though a solid evidence base is crucial in any area, in tobacco the need for unbiased evidence is particularly strong. Competing interests, including those of the tobacco industry, can heavily influence publications. By systematically collecting evidence, assessing it for methodological issues, and implementing thorough analyses, TAG has amassed unbiased and comprehensive bodies of evidence for life-saving interventions. These reviews are regularly updated, which makes them dynamic and able to account for developments in the literature.
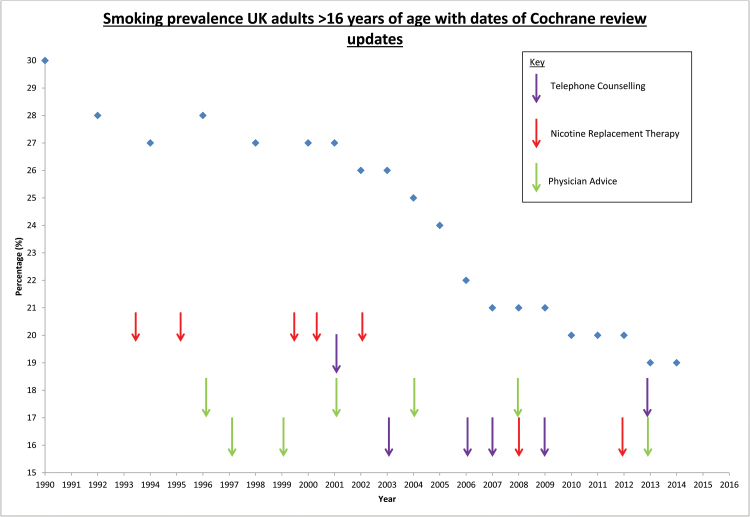
Smoking prevalence has fallen in the United Kingdom since TAG was formed (see Figure 1), though the direct relationship between guidelines, practice, and smoking prevalence cannot be quantified. When the group began, the prevalence of smoking in the adult population of the United Kingdom was 28%.16 In 1998, the UK government published its white paper “Smoking Kills,”17 which sets out a range of interventions for reducing smoking prevalence in the United Kingdom and included a substantial investment in smoking cessation services. This white paper was based on UK national guidelines, which drew on evidence from Cochrane TAG’s reviews. In the subsequent years, the prevalence of adult smoking in the United Kingdom has fallen below 20%, and there are over two million fewer adult smokers in the United Kingdom.16 There is substantial evidence of the impact of TAG’s research on the policies that have been implemented during this period, and the following section highlights the use of three Cochrane reviews in guidelines and practice shaping documents.
Figure 1.
Graph of smoking prevalence in the United Kingdom alongside key Cochrane Tobacco Addiction Group review updates.
Case Studies: Findings and Impact
The following case studies of Cochrane TAG reviews give an idea of the work carried out by the group. All three were written by members of the group’s editorial base: one of nicotine replacement therapy (NRT); one of physician advice; and one of telephone counseling, all for smoking cessation.
Nicotine Replacement Therapy (First Published in 1996)
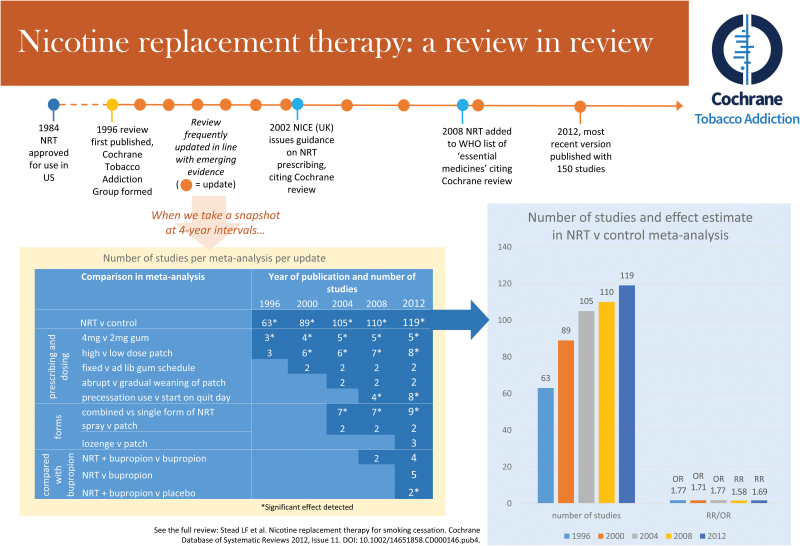
Since the first version of the Cochrane review of NRT, this review has been updated regularly (see Figure 2). The first version included 72 studies; it now contains 150. This review investigates different forms, delivery methods and settings, schedules, and dosages of NRT in a range of subgroups and shows definitively that all commercially available forms of NRT help people to quit smoking relative to placebo or no NRT control (risk ratio [RR] = 1.60, 95% confidence interval [CI] = 1.53–1.68).18
Figure 2.
Illustration of the key characteristics and updates of the Cochrane Tobacco Addiction Group nicotine replacement therapy review. Figure is also available at https://www.phc.ox.ac.uk/images/misc/cochranenrtinfographic.
This review provided evidence for a 2008 proposal for the inclusion of NRT in the World Health Organization (WHO) list of essential medicines, which described the review as “the largest database on the effectiveness of NRT.”19 This proposal was approved in May 2009. The recommendation was said to be supported by Cochrane TAG’s “high-quality evidence of effectiveness.”20 WHO predicted that the inclusion of NRT on the list of essential medicines would advance guideline development and improve access to NRT in developing countries.20
Beyond international use, the NRT review has been cited in three key national UK guidelines in recent years, which continue to shape practice. The evidence review21 underpinning NICE guidance on Brief Interventions and Referral for Smoking Cessation recommends the use of NRT based on the Cochrane review. Guidelines published from the United States22 and Australia23 also cite the review, using it as consistent evidence for use of NRT as a medication to aid smoking cessation and using it to support the specific recommendation that heavily dependent smokers use higher doses of oral NRT. In the case of US guidelines,22 the review was cited alongside an extensive meta-analysis conducted by guideline developers.
In addition to its use in guidelines, the NRT review has also recently been used to inform point of care material24 and patient information sources25 in the United Kingdom.
Physician Advice (First Published in 1996)
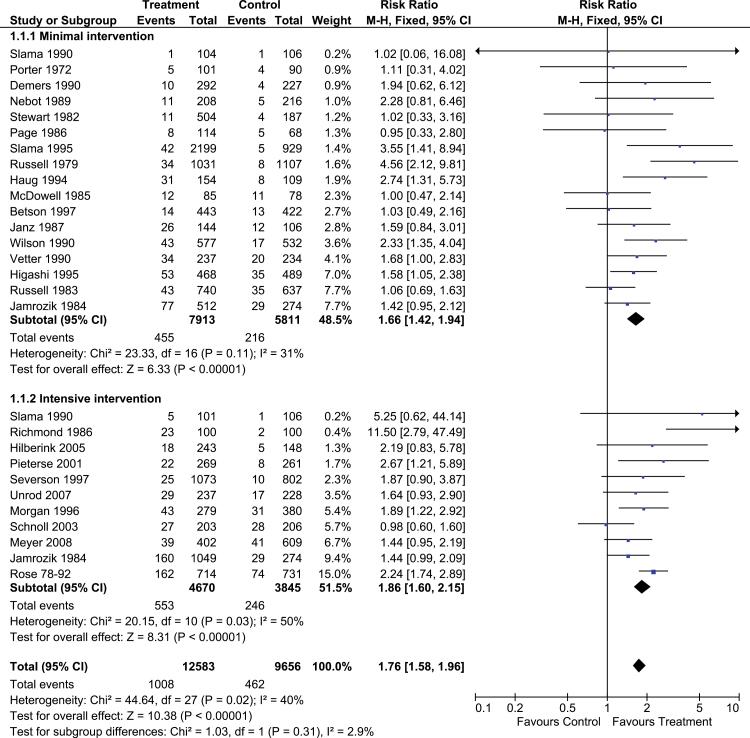
This review has been updated eight times since first publication, currently includes 41 trials, and demonstrates that brief clinical advice is associated with a significant increase in the rate of quitting (RR = 1.66, 95% CI = 1.42–1.94), in comparison to no advise or usual care. The association was found to be dose responsive—stronger with more intensive advice (see Figure 3).13
Figure 3.
A forest plot of the effect of physician advice for smoking cessation versus control (subgroups by intensity of advice); outcome: smoking cessation at longest follow-up.
Many physicians were initially reluctant to advise patients not to smoke, as the efficacy of the approach was doubted and there were concerns it would affect the doctor–patient relationship. However, the Cochrane TAG review of physician advice for smoking cessation has been used in numerous guidelines as definitive evidence for the efficacy of this approach, and has shaped practice accordingly.
Australian guidelines23 use the review to support the assertion that all health professionals should systematically identify smokers and offer them treatment advice at every opportunity, using the summary statistic from the review in support of brief motivational advice. In a table listing barriers to health professionals providing smoking cessation services, one of the seven barriers listed is the belief, “I am not effective.” The Cochrane review is cited as evidence that this is not the case. US guidelines22 and the NICE Rapid Review of Brief Interventions and Referral for Smoking Cessation cite the review as evidence that brief advice from a physician is effective21 and is consistent with the meta-analysis conducted by US guideline developers on the topic.22
Telephone Counseling (First Published in 2000)
This review has been updated six times since first publication and contains 65 trials. Higher quit rates were found in participants randomized to multiple sessions of proactive counseling (RR = 1.38, 95% CI = 1.28–1.49), and telephone counseling not initiated by calls to helplines was also shown to increase quit rates (RR = 1.29, 95% CI = 1.20–1.38).26
Quitlines are widely available throughout North America, Europe, and Asia and are a major area of public health investment in many countries. TAG’s review of telephone counseling has informed the current and continued funding of these quitlines. The review has also informed specific aspects of telephone counseling methods.
Australian guidelines23 cite the review in support of the use of proactive calls and provide summary statistics to illustrate the overall efficacy of telephone counseling. In the evidence review for NICE’s guidelines on the Impact of Quitlines on Smoking Cessation,27 they report that Cochrane TAG’s review “provided a key source of information” for the guidelines and it is cited as strong evidence for both proactive phone counseling and additional sessions of phone counseling. In addition, US guidelines22 use the review as the sole source of evidence that quitlines significantly increase abstinence rates.
These examples are by no means comprehensive and represent only three of a body of around 70 reviews. These reviews have been used to shape policy and practice not only in the United Kingdom, the United States, and Australia but also in countries as diverse as Canada, Finland, Paraguay, Portugal, and Uruguay. In addition, the UK National Centre for Smoking Cessation and Training use a variety of Cochrane TAG’s reviews to inform the training that they provide for smoking cessation practitioners.
New Evidence
The Case Studies section provides information on TAG’s long-term achievements and contributions; however, with the ever-changing landscape of tobacco addiction treatment, and the evolving treatment needs of those who continue to smoke, the landscape of research into tobacco control is still changing. It is vital that TAG’s reviews accommodate this to ensure that they remain useful and valid.
A prime example of this was the recent publication (2014) of a new review for the group, looking at the effects of electronic cigarettes (EC) on smoking reduction and cessation.28 The review was the first attempt made to systematically review the literature on this topic. In doing so, it highlighted the small amount of high-quality evidence evaluating EC (with only two randomized controlled trials meeting the criteria for inclusion), despite an enormous increase in their use and in interest and discussion on the subject in recent years. The review found that, “Participants using an EC were more likely to have abstained from smoking for at least six months compared with participants using placebo EC (RR 2.29, 95% CI 1.05 to 4.96; placebo 4% versus EC 9%; 2 studies”).28 However, this needs to be considered alongside the fact that the authors rated the quality of the evidence as “Low” by GRADE standards,29 due to imprecision as a result of the small number of trials. This means that further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate in the future. Despite this, the review garnered a great deal of publicity worldwide, following a press release and a news briefing by the authors at the Science Media Centre in London, UK. The review is very likely to influence future health care guidance, as it is currently one of the only sources of systematic, high-quality evidence on a high-profile subject. The next update of the review is planned for 2016.
Similarly, the group recently aided the update of a review of legislative smoking bans on health outcomes,30 which also attracted a lot of media attention—again largely because it is the result of the most thorough attempt to systematically review the topic to date. Unlike many of the reviews published by Cochrane TAG, all of the evidence presented in this review is from observational studies. This makes it very difficult to account for potential confounding, but even taking this into consideration, it was concluded that there was moderately strong evidence to conclude that smoking bans lead to a reduction in heart disease and deaths from smoking-related illnesses, and that the greatest drop in hospital admissions for heart disease was in populations of nonsmokers. This information is particularly important in the context of public health policy decisions and is a key illustration of how Cochrane reviews can be useful in this regard, and not just in informing clinical and personal treatment decisions.
Moving Forward
Cochrane TAG intends to move forward following three distinct workstreams, outlined in the following sections. These directions have been chosen to maintain the group’s momentum and to ensure the group continues to contribute to developments in the field.
Adapting Updates of Reviews by Responding to Changes in the Use of Existing Treatments
One of the strengths of the Cochrane method is the development and publication of review protocols. This ensures transparency and therefore minimizes bias. However, it is also important to adapt to developments in the field. For example, NRT was originally used in singular form; however, it is now common to use it in combination (eg, a patch in combination with a short acting form, such as gum). It was important that the review accommodated this change in order to inform clinicians and the public about the safety and effectiveness of using NRT in this way. As long as any changes to methods remain transparent and are explained in the update, this is essential to keeping Cochrane reviews relevant.
Developing New Protocols and Publishing New Reviews to Further Widen the Cochrane Evidence Base
Although TAG can identify necessary topics for review and either carry these out themselves or contact potential external author teams, existing or potential authors are encouraged to liaise with Cochrane TAG about any ideas they may have for new reviews they would like to carry out (see Box 4 for more information on how to get involved). The group has an editorial base that can then discuss the proposal and ensure that it is relevant to the group, that it is not covered by an existing review, and that the author team has the necessary expertise to carry out the review, with support. This takes into account that Cochrane themselves offer training in their methods, and so it is not imperative that proposed authors have carried out a Cochrane Review before. Through working with external author teams, Cochrane TAG plans to publish a number of new reviews in the next year, including investigations of institutional smoking bans, tobacco packaging design, and treatment in primary care and psychiatric settings.
Box 4. How You Can Get Involved
Comment on our reviews—register with our group to review our outputs before they come out (e-mail Cochrane.tobacco@phc.ox.ac.uk).
Help identify areas where we should be conducting reviews—as an author if you like! (e-mail Cochrane.tobacco@phc.ox.ac.uk).
Help identify reports of randomized controlled trials through Cochrane Crowd (http://crowd.cochrane.org/faq.html).
Participate in other tasks (eg, translation) through Cochrane Task Exchange (http://taskexchange.cochrane.org/).
If you are a patient, carer, or member of the public, consider joining the Cochrane Consumer Network (Consumers.Cochrane.org). This is based in over 79 countries and provides training and support to help consumers identify research priorities, work alongside researchers to produce reviews, and check the readability and help to write plain language summaries.
Speaking to Our Stakeholders
Until now, TAG’s review portfolio has been shaped by researchers. However, this year, as part of our 20th anniversary celebrations, we proposed to speak to our stakeholders through a priority setting exercise, involving public and stakeholder dialogue. A two-part survey on questions that still need to be answered in tobacco control was distributed to policy makers, health care providers, smokers, former smokers, guideline developers, researchers, and research funders, February to May 2016. This was followed by a 1-day workshop in June, during which the review portfolio was reviewed in light of the previously identified questions and future priorities set—for new reviews, updating existing reviews, and considering ways in which the TAG portfolio could better meet the needs of a broader user group. Broadening input will ensure reviews meet a wide range of needs, are relevant to current trends in smoking, and therefore create the highest possible impact, while guarding against research wastage. The James Lind Alliance advocates such an approach, and we drew on their key principles to pioneer this inclusive methodological approach to priority setting. The results of this exercise will be written up for peer-reviewed publication and presented, and the group will begin work on the priorities identified.
Conclusion
In conclusion, Cochrane TAG’s work investigates and summarizes the literature base for interventions aiming to prevent or treat tobacco addiction, focusing on the areas that will be most relevant to users. In doing so, reviews to date have had a wide and deep impact across guideline development and treatment services. The group’s aims going forward are to ensure that this continues and to adapt to emerging trends. As time goes on this will become a less straightforward task, and therefore, it makes sense for TAG to harness the ideas of the people they wish to help and ask them what is important to them. With finite resources, it is important to remain focused on relevant questions to drive the field forward and assist in reducing and treating tobacco use.
Funding
The Cochrane Tobacco Addiction Group is funded by the National Institute for Health Research (NIHR). The Cochrane TAG Twentieth Anniversary Priority Setting (CTAG taps) project is funded by the NIHR School for Primary Care Research. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Declaration of Interests
NLH is coinvestigator on a trial testing nicotine patch preloading, funded by the NIHR, Health Technology Assessment Programme. The pharmacotherapy used in the intervention arm of this trial was provided free of charge by GlaxoSmithKline plc. None of the trial investigators are directly employed by GlaxoSmithKline plc, and GlaxoSmithKline plc had no involvement in the design, conduct, or analysis of the trial. JHB and LH have no declarations of interest.
Acknowledgments
We gratefully acknowledge Cochrane Tobacco Addiction Group (TAG) funding from the NIHR, as well as all employees and contributors to the work of Cochrane TAG past and present.
References
- 1. ASH. Smoking and disease http://ash.org.uk/information-and-resources/fact-sheets/smoking-and-disease/. Published 2015. Accessed May 23, 2016.
- 2. ASH. The economics of tobacco http://ash.org.uk/information-and-resources/fact-sheets/the-economics-of-tobacco/. Published 2015. Accessed May 23, 2016.
- 3. Guyatt GH. The way of the past. ACP J Club. 1991;114(2):A16. [Google Scholar]
- 4. Sackett DL. Clinical epidemiology. Am J Epidemiol. 1969;89(2):125–128. [DOI] [PubMed] [Google Scholar]
- 5. Feinstein AR, Di Massa R. Prognostic significance of valvular involvement in acute rheumatic fever. N Engl J Med. 1959;260(20):1001–1007. [DOI] [PubMed] [Google Scholar]
- 6. Feinstein AR. Clinical epidemiology: I. The populational experiments of nature and of man in human illness. Ann Intern Med. 1968;69(4):807–820. [DOI] [PubMed] [Google Scholar]
- 7. Feinstein AR. Clinical epidemiology: II. The identification rates of disease. Ann of Intern Med. 1968;69(5):1037–1061. [Google Scholar]
- 8. Feinstein AR. Clinical epidemiology: III. The clinical design of statistics in therapy. Ann Intern Med. 1968;69(6):1287–1312. [DOI] [PubMed] [Google Scholar]
- 9. Cochrane AL. Sickness in Salonica: my first, worst, and most successful clinical trial. Br Med J (Clin Res Ed). 1984;289(6460):1726–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cochrane A, Cox JG, Jarman TF. Pulmonary tuberculosis in the Rhondda Fach. Br Med J. 1952;2(4789):843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chalmers I, Dickersin K, Chalmers TC. Getting to grips with Archie Cochrane’s agenda. Br Med J. 1992;305(6857):786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chalmers I, Enkin M, Keirse MJNC. Effective Care in Pregnancy and Childbirth. Oxford, UK: Oxford University Press; 1989. [Google Scholar]
- 13. Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;5:CD000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White AR, Rampes H, Liu JP, Stead LF, Campbell J. Acupuncture and related interventions for smoking cessation. Cochrane Database Syst Rev. 2011;1:CD000009. [DOI] [PubMed] [Google Scholar]
- 15. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database of Syst Rev. 2013;5:CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. ASH. Smoking statistics who smokes and how much http://ash.org.uk/information-and-resources/fact-sheets/smoking-statistics-who-smokes-and-how-much/. Published 2016. Accessed May 23, 2016.
- 17. Government. Smoking kills: a white paper on tobacco https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/260754/4177.pdf. Published 1998. Accessed September 9, 2016.
- 18. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. [DOI] [PubMed] [Google Scholar]
- 19. WHO Expert Committee. The Selection and Use of Essential Medicines. Geneva, Switzerland: World Health Organization; 2008. World Health Organization Technical Report Series No. 950. [Google Scholar]
- 20. WHO. Two forms of nicotine replacement therapy chosen as WHO “Essential Medicines.”http://www.who.int/tobacco/communications/highlights/note_nrt_therapy/en/. Published 2009. Accessed September 9, 2016.
- 21. Stead L, McNeill A, Shahab L, West R. Rapid Review of Brief Interventions and Referral for Smoking Cessation. London, UK: NICE; 2006. Public Health Guidance PH1. [Google Scholar]
- 22. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; https://www.ncbi.nlm.nih.gov/books/NBK63952/. Published May 2008. Accessed May 16, 2016. [Google Scholar]
- 23. Zwar N, Richmond R, Borland R, et al. Supporting Smoking Cessation: A Guide for Health Professionals. South Melbourne, Victoria, Australia: Royal Australian College of General Practitioners; 2011. http://whyquit.com/guidelines/2011_Australia_Guide.pdf. [Google Scholar]
- 24. NICE. Clinical Knowledge Summaries. Smoking cessation: evidence http://cks.nice.org.uk/smoking-cessation#!supportingevidence1. Published 2012. Accessed May 16, 2016.
- 25. patient.co.uk. Nicotine replacement therapy http://www.patient.co.uk/health/Smoking-Nicotine-Replacement-Therapy.htm. Accessed May 16, 2016.
- 26. Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2013;8:CD002850. [DOI] [PubMed] [Google Scholar]
- 27. Bell K, Richardson L, Greaves L. The impact of quitlines on smoking cessation (NICE Rapid Review). https://www.nice.org.uk/guidance/ph10/evidence/the-impact-of-quitlines-on-smoking-cessation-369844669. Published 2007. Accessed May 16, 2016. [Google Scholar]
- 28. McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;12:CD010216. [DOI] [PubMed] [Google Scholar]
- 29. Guyatt GH, Oxman AD, Schünemann HJ. GRADE guidelines-an introduction to the 10th-13th articles in the series. J Clin Epidemiol. 2013;66(2):121–123. [DOI] [PubMed] [Google Scholar]
- 30. Frazer K, Callinan JE, McHugh J, et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst Rev. 2016;2:CD005992. [DOI] [PMC free article] [PubMed] [Google Scholar]