Scheme 3.
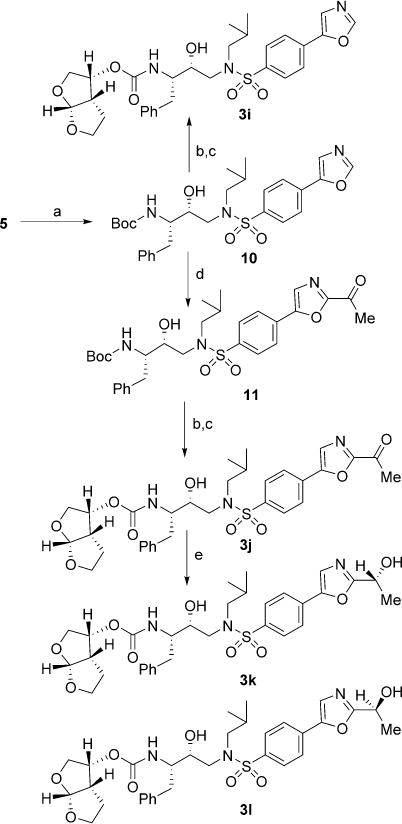
Reagents and conditions: a) TosMIC, K2CO3, MeOH, 55°C, 1.5 h, 96%; b) TFA, CH2Cl2, 23°C, 2.5 h; c) 6, N,N-DIPEA, MeCN, 23°C, 4–7 days, 47–80% (over two steps); d) iPrMgCl, dry THF, 15°C, 40 min, then AcN(-Me)OMe, −15°C→23°C, 5 h, 79%; e) RuCl[(−R,R)-TsDPEN](mesitylene) for 3k or RuCl(p-cymene)[(S,S)-Ts-DPEN] for 3l, TEA, HCOOH, dry CH2Cl2, 0°C→23°C, 7 h, 95–97%.
