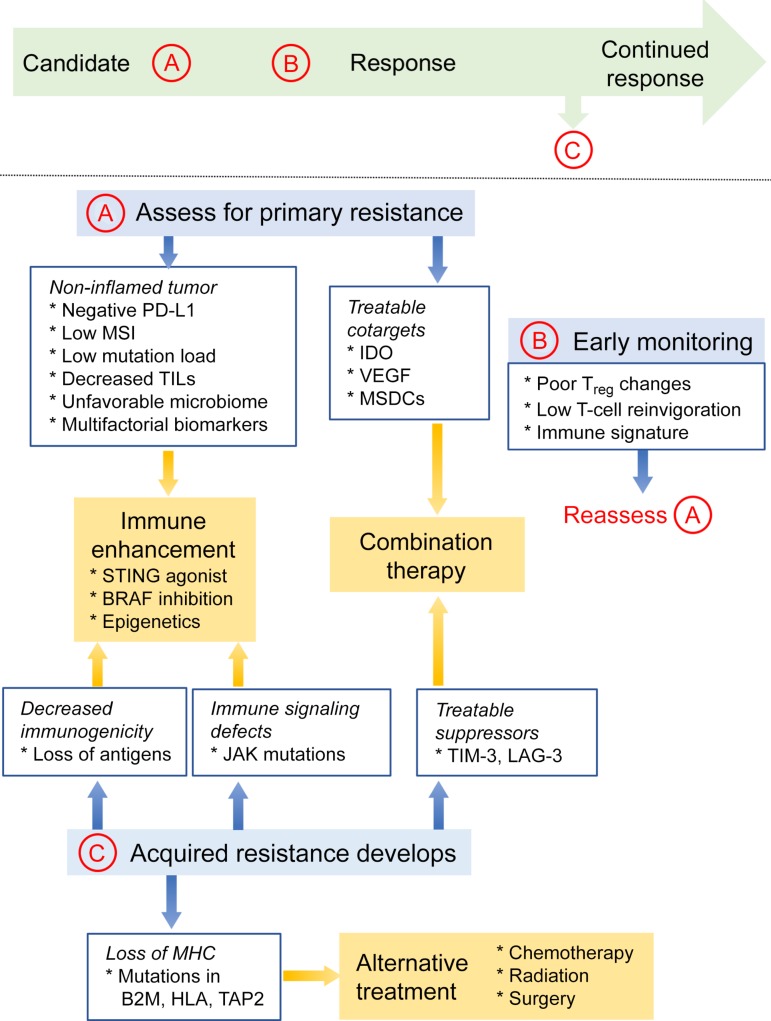
Figure 3.
Proposed evaluation of prospective candidates for immunotherapy. Candidate patients should undergo an initial evaluation for resistance biomarkers (A). A clinical profile consistent with a noninflamed tumor microenvironment may warrant enhancement of immune activity. Biomarkers that can be targeted would suggest benefit from combination therapies. Primary resistance might also be suggested by negative prognostic indicators during early on‐treatment (B). Patients whose disease progresses after initial response should receive workup for acquired resistance (C). These resistance mechanisms can similarly be addressed by enhancing the immune response or targeting immunosuppressive pathways with combination therapy. Finally, loss of MHC‐I may prohibit further use of immunotherapy and indicate alternative modalities.
Abbreviations: B2M, beta‐2 microglobulin; HLA, human leukocyte antigen; IDO, indoleamine 2,3‐dioxygenase; JAK, Janus kinase; LAG‐3, lymphocyte‐activation gene‐3; MDSCs, myeloid‐derived suppressor cells; MHC‐I, major histocompatibility class I; MSI, microsatellite instability; PD‐L1, programmed cell death ligand 1; STING, stimulator of interferon genes; TAP2, transporter associated with antigen processing 2; TIL, tumor‐infiltrating lymphocytes; TIM‐3, T‐cell immunoglobulin mucin‐3; Treg, regulatory T cells; VEGF, vascular endothelial growth factor.