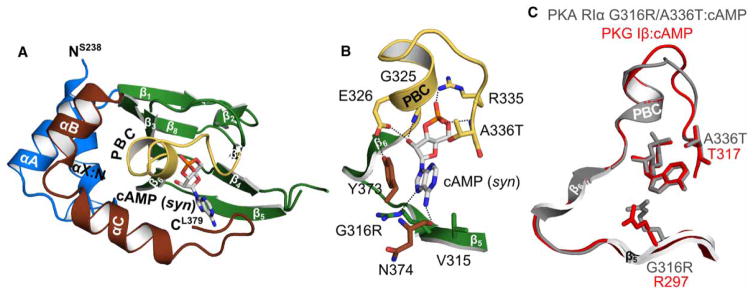
Figure 5. Crystal structure of PKA hRIα CNB-B (234–381) G316R/A336T bound with cAMP.
(A) Overall structure of the PKA RIα CNB-B G316R/A336T double mutant bound with cAMP. The protein is shown in cartoon representation with the secondary structure elements labeled, while cAMP is shown as sticks. (B) Detailed view of the CNB pocket with the bound cAMP. The introduced A336T does not interact with cAMP. The side chain of G316R moves away from the cAMP, indicating a steric clash between the position 6 amino group and the arginine side chain. Therefore, R316 is a negative determinant for binding of cAMP as recently described for PKG Iβ [21]. However, a hydrogen bond with the N7 of the purine ring can still be formed. Residues are labeled and hydrogen bond interactions are shown as dashed lines. (C) Structural alignment of the PKA RIα CNB-B G316R/A336T: cAMP complex (gray) with the PKG Iβ CNB-B: cAMP complex structure (red; PDB code 4QX5) [21].