Table 3.
Scope of mesolytic bond cleavage induced nucleophilic substitution.a
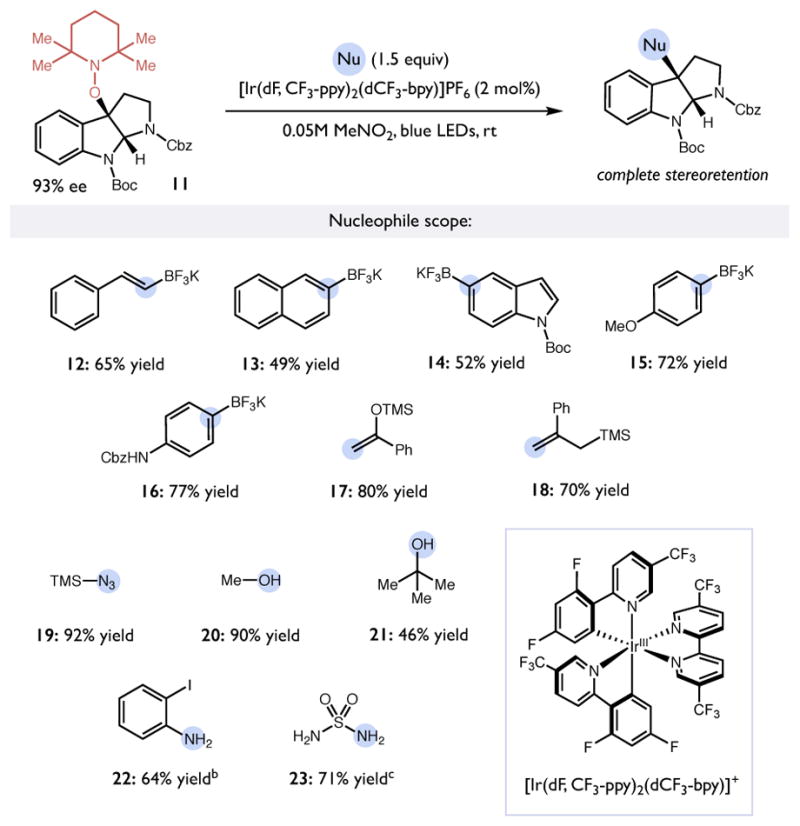
|
Reactions run on a 0.25 mmol scale. Reported yields are for isolated and purified materials. The optical purity of all products was confirmed by HPLC analysis.
Unprotected pyrroloindoline 2 as the substrate.
Conducted with 6 equiv. of sulfamide.
