Table 4.
Optimization of heterodimerizationa and synthesis of (−)-calycanthidine.
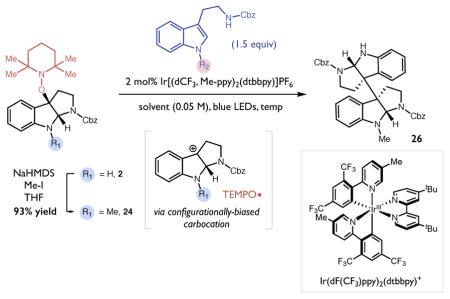
| |||||||
|---|---|---|---|---|---|---|---|
| Entry |
|
|
solvent | additive | temp | % yield | dr (C2:meso) |
| 1 | H | Me | THF | — | rt | 21 | 1:1 |
| 2 | H | Me | THF | — | −40 ºC | 10 | 2:1 |
| 3 | Me | H | CH2Cl2 | — | rt | 48 | 5:1 |
| 4 | Me | H | CH2Cl2 | — | −40 ºC | 30 | 14:1 |
| 5 | Me | H | CH2Cl2 | TFA (1 equiv) | −40 ºC | 71 | 14:1 |
| Diastereoselective synthesis of (−)-calycanthidine | |||||||
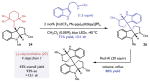
| |||||||
Optimization reactions performed on a 0.025 mmol scale. Yields and diastereomeric ratios were determined by 1HNMR analysis of the crude reaction mixtures. Optimized heterodimerization reaction performed on 2 mmol scale.
