Fig. 8.
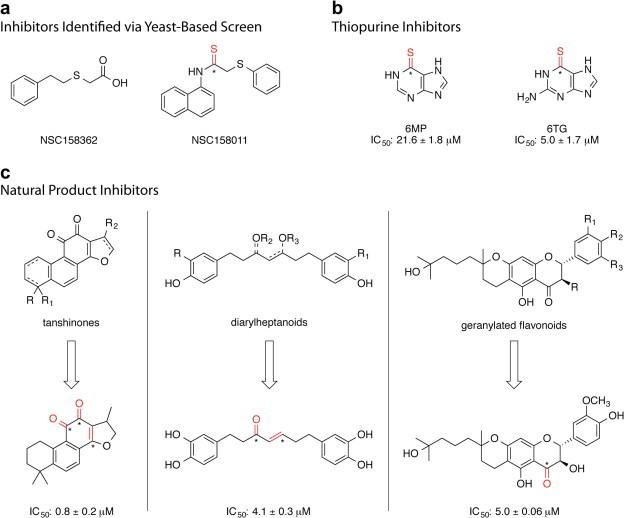
SARS-CoV PLpro inhibitors identified through small-scale screening efforts. Positions within each molecule that are susceptible to covalent modification through nucleophilic attack (“warhead” positions) are indicated in red where the electrophilic carbons are indicated with an asterisk. (a) Inhibitors identified via yeast-based screen (Frieman et al., 2011), (b) thiopurine inhibitors (Chen et al., 2009, Chou et al., 2008), (c) natural product inhibitors (Cho et al., 2013, Park et al., 2012a, Park et al., 2012b), where the most potent natural product of each class is shown and the IC50 detailed.
