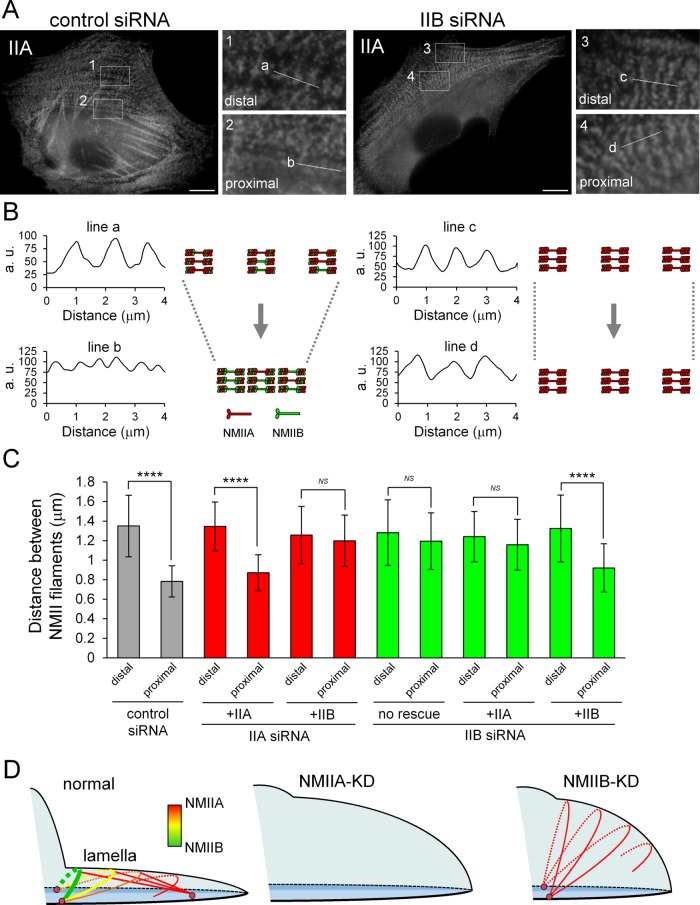
FIGURE 4:
Both NMIIA and NMIIB are required for normal contraction of TAs. (A) SV1 cells treated with the indicated siRNAs were fixed and stained with an Alexa Fluor 596–conjugated anti–NMHC-IIA polyclonal antibody. Enlarged images of the boxed regions in the overall image showing the central portion of TAs. Images were captured using a conventional fluorescence microscope. Bar, 10 μm. (B) Scans along the white lines in A showing the distances between the stacks of NMII filaments in TAs. The schematic illustrations indicate the contraction process of TAs in each condition. (C) Quantification of the distance between NMII filaments in each condition (n > 30 pitches from >5 cells/condition). The distances between NMII filaments were measured by the RGB Profile plot plug-in of ImageJ software. ****p < 0.00005. Note that the distance between stacks was not decreased in NMIIB-KD cells during centripetal flow. (D) Model for the role of TAs in lamellar flattening. Schematic illustration depicting the lamellar shape of each siRNA-treated cell. Arcs, straight lines, and pink circles indicate TAs, dSFs connecting to TAs at right angles, and FAs, respectively. Red and green correspond to NMIIA and NMIIB in the SF subtypes, respectively. TAs form via the association of NMIIA with actin filaments in the distal region of the lamella and are then transferred to the cell body. During centripetal flow, TAs link to FAs derived from the distal end of dSFs at both ends (Hotulainen and Lappalainen, 2006), as well as to dSFs connecting to TAs at right angles, and then NMIIB is incorporated into TAs. TAs do not form in NMIIA-KD cells. The flattened lamella is maintained by the contraction of TAs. The tension generated by this contraction is transmitted to FAs at the distal end of dSFs (Burnette et al., 2014). Thus, lamellar flattening is affected in both NMIIA-KD and NMIIB-KD cells due to the lack of TAs and the absence of functional TAs, respectively.