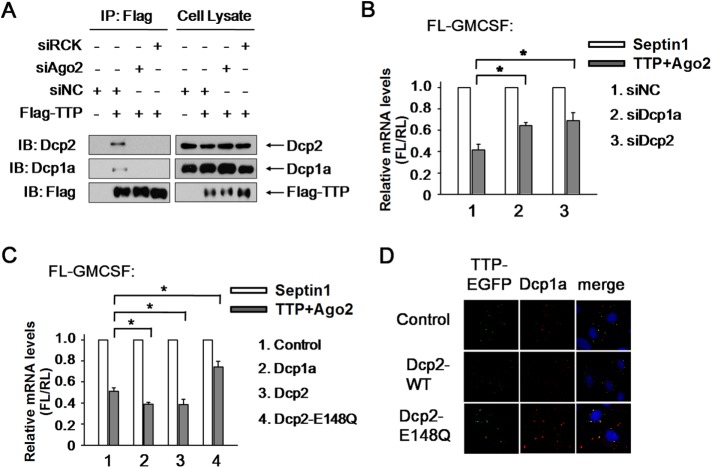
FIGURE 5:
Dcp1a and Dcp2 are responsible for TTP- and Ago2-mediated ARE-mRNA decay. (A) Western blots for the endogenous Dcp1a and Dcp2 proteins that coimmunopurify with Myc-tagged TTP from RNase A–treated HEK 293T cells treated with Scrambled siRNA, siAgo2, or siRCK. Precipitates (left panels) and total extracts (input, right panels) were probed for the presence of Dcp1a, Dcp2, and TTP as indicated. (B) 293T cells were transfected with the FL-GM-CSF reporter and RL plasmids, together with siRNA as indicated. Flag-tagged TTP and Myc-tagged Ago2 were also included in the transfection mixture. Flag-tagged Septin1 and Myc-tagged Septin1 served as control. The relative value of FL mRNA level was set to 1 for cells transfected with the plasmid expressing Septin1 in each knockdown condition. Means and SD from three independent experiments are shown. *p < 0.05. (C) 293T cells were transfected with the FL-GM-CSF reporter and RL plasmids, together with plasmid expressing HA-tagged Dcp1a, Dcp2, Dcp2-E148Q, or Septin1. Plasmid expressing Flag-tagged TTP and Myc-tagged Ago2 were also included in the transfection mixture. Flag-tagged Septin1 and Myc-tagged Septin1 served as control. The relative value of FL mRNA was set to 1 for cells transfected with the Flag-tagged and Myc-tagged Septin1 plasmids in each condition. Means and SD from three independent experiments are shown. *p < 0.05. (D) Dcp2-E148Q mutant significantly retains TTP localization in PBs. HeLa cells were transfected with Myc-tagged wild-type Dcp2 (Dcp2-WT), Dcp2-E148Q mutant, or the corresponding empty expression plasmid (control), together with plasmid TTP-EGFP. PBs were shown by anti-Dcp1a staining.