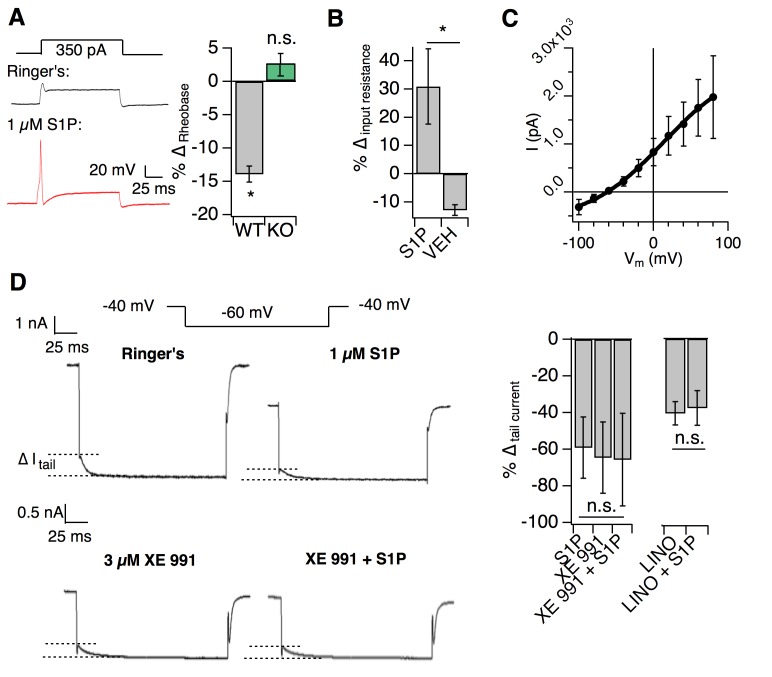
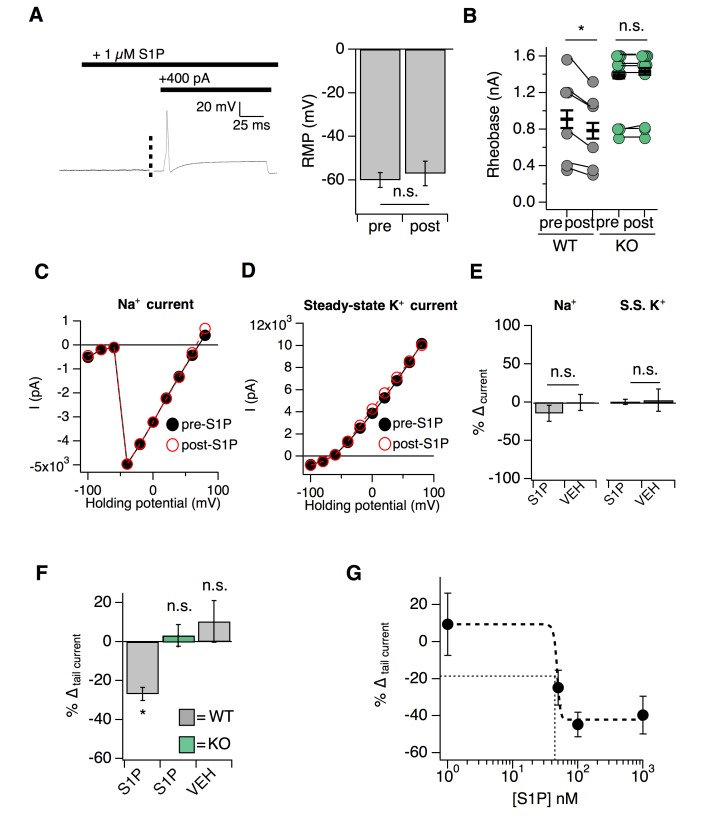
Figure 5. S1PR3 modulates KCNQ2/3 channels to regulate AM excitability.
All experiments were performed in S1pr3mCherry/+ or -/- DRG neurons. (A) (Left) Example traces of a single mCherry +neuron in whole cell current clamp before and after S1P application. (Right) % change in rheobase after S1P application for S1pr3mCherry/+ (left, n = 7) and KO (right, n = 12) neurons (pWT,KO = 0.012, 0.287; two-tailed paired t-tests). (B) % ∆ in input resistance after S1P or vehicle application (p=0.017; two-tailed paired t-test; n = 4 cells per group). (C) The S1P-sensitive current is carried by potassium. The current-voltage relationship was determined by subtraction of the post-S1P current from the pre-S1P current and reverses at −60.125 mV; n = 6 cells. Data were fitted with a Boltzmann equation. Pre- and post-S1P currents were measured at the indicated voltage (−100 mV to +80 mV, 20 mV increments) following a +100 mV step (100 ms). Current was quantified using the peak absolute value of the slowly-deactivating current 0–10 ms after stepping to indicated voltage. Unless indicated otherwise, all error bars represent mean ± SEM. (D) (Graphic, top) Averaged current traces of a single mCherry+ neuron in whole cell voltage clamp recording comparing tail currents (∆I tail) pre- and post-S1P using indicated voltage step protocol. (graphic, bottom) Averaged current traces of a single mCherry+ neuron in whole cell voltage clamp recording with XE991 treatment. Holding phase (−40 mV, 150 ms) was truncated in traces. (Left graph) % ∆ in outward tail current (average ±SD after indicated treatments (1 µM S1P, 3 µM XE 991, or both) for S1pr3mCherry/+ medium-diameter neurons; (p=0.58; one-way ANOVA; n = 6, 8, 14 cells) using protocol depicted at right. (Right graph) % ∆ in inward tail current after indicated treatments (LINO = 100 µM linopirdine) for S1pr3mCherry/+ medium-diameter neurons; (p=0.47; two-tailed paired t-test; n = 12 cells).