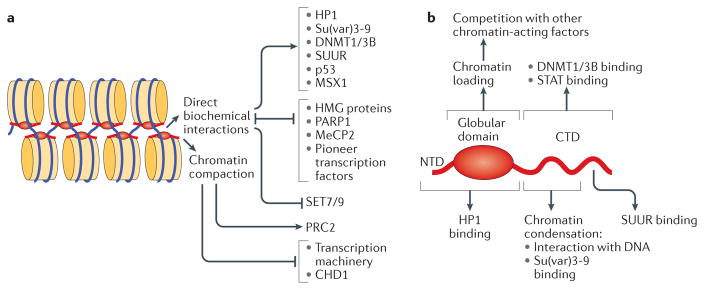
Figure 5. Biochemical activities of linker histones.
a | Alternative molecular mechanisms used by linker histones (H1) to modulate the activity of chromatin. Direct biochemical interactions with H1 facilitate or inhibit chromatin binding of various structural proteins, enzymes and transcription factors; in addition, direct competition mechanisms control the mutually exclusive distribution patterns of H1 with various chromatin-interacting proteins. In general, H1-dependent chromatin compaction interferes with transcription initiation by preventing nucleosome remodelling and the binding of sequence-specific transcription factors, as well as the binding and translocation of general transcription factors. However, it has been shown that the compacted chromatin state established by H1 stimulates the activity of Polycomb repressive complex 2 (PRC2). b | Structural domains of the H1 polypeptide involved in H1 deposition, physical interactions and regulatory functions. Distinct regions within the globular domain and the carboxy-terminal domain (CTD) mediate the multiple biochemical activities of H1. CHD1, chromodomain-helicase-DNA-binding protein 1; HMG, high-mobility group; HP1, heterochromatin protein 1; MeCP2, methyl-CpG-binding protein 2; NTD, amino-terminal domain; PARP1, poly(ADP-ribose) polymerase 1; STAT, signal transducer and activator of transcription; SUUR, suppressor of underreplication.