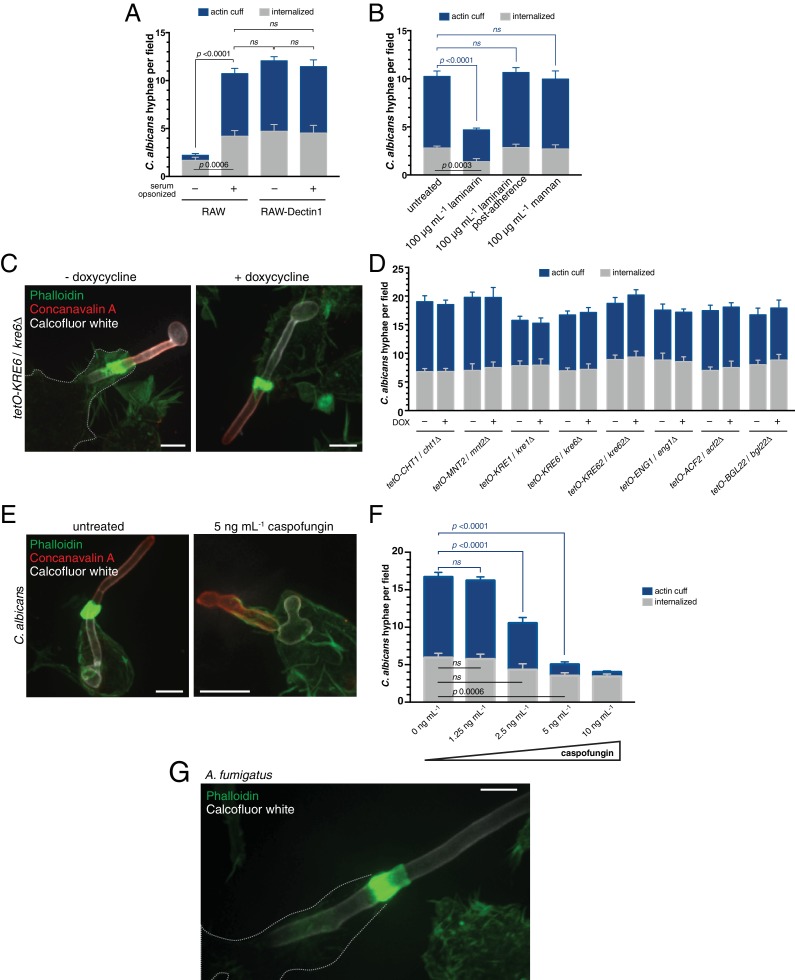
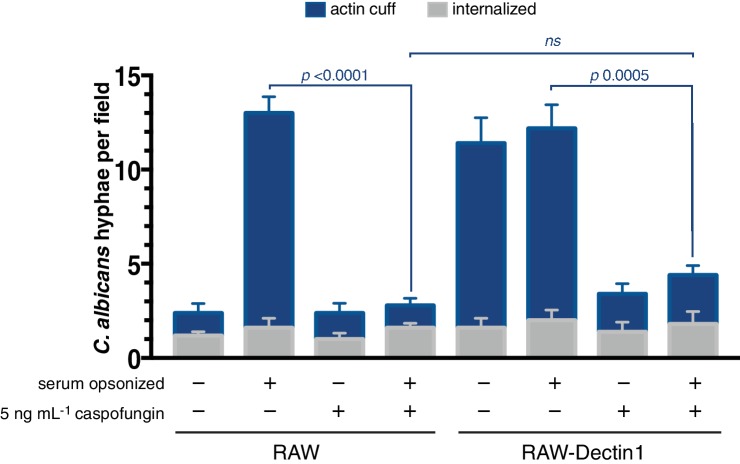
Figure 4. Assessing the contribution of C. albicans cell wall components to actin cuff formation.
(A) RAW or RAW-Dectin1 cells were incubated with Candida-BFP hyphae that had been either untreated or serum-opsonized. Following phagocytosis, extracellular C. albicans was stained using concanavalin A, and actin stained with phalloidin. The number of C. albicans hyphae that were fully internalized or partially internalized with actin cuffs per 37.5x field was counted by confocal microscopy. Average number of C. albicans per field was 15.7 ± 1.3. For each condition, three independent experiments were quantified, with ≥4 fields counted per replicate. p value was calculated using the unpaired, 2-tailed students t-test. Data are means ±SEM. (B) RAW-Dectin1 cells were allowed to internalize Candida-BFP hyphae in the presence or absence of laminarin or mannan. For laminarin, RAW-Dectin1 cells were also allowed to adhere C. albicans 15 min prior to the addition of laminarin, as indicated. Other details as in A. Average number of C. albicans per field was 12.9 ± 0.7. (C–D) Evaluation of C. albicans GRACE strain cell wall mutants for actin cuff formation. GRACE strains were induced to form hyphae in the absence or presence of doxycycline (DOX) to repress target gene expression, and incubated with RAW-Dectin1 cells. Following phagocytosis, monolayers were fixed and C. albicans stained with 10 μg mL−1 calcofluor white (white), extracellular C. albicans stained using concanavalin A (red), and actin stained with phalloidin (green). Image in C is representative of ≥30 fields from ≥3 separate experiments of each type. Scale bar: 5 μm. (D) The number of C. albicans hyphae that were fully internalized or partially internalized with actin cuffs per 37.5x field was counted by confocal microscopy, and the average number per field calculated. Average number of C. albicans per field was 20.6 ± 0.6. For each condition, three independent experiments were quantified, with ≥4 fields counted per replicate. p value was calculated using the unpaired, 2-tailed students t-test. Data are means ±SEM. (E) Role of C. albicans β-(1,3)-glucan in actin cuff formation. The GRACE wild-type strain was incubated and induced to form hyphae in the presence or absence of 5 ng mL−1 caspofungin and incubated with RAW-Dectin1 cells for phagocytosis. Following phagocytosis, cells were fixed and C. albicans stained with 10 μg mL−1 calcofluor white (white), extracellular C. albicans stained using fluorescent concanavalin A (red), and actin stained with fluorescent phalloidin (green). Image is representative of ≥30 fields from ≥3 separate experiments. Scale bar: 5 μm. (F) The effect of β-(1,3)-glucan synthase inhibition on actin cuff formation. Hyphae were prepared as in (E), with varying concentrations of caspofungin, as indicated. Phagocytosis, fixation and staining as in (E). Other details as in (A). Average number of C. albicans per field was 19.7 ± 0.8. (G) Actin cuffs are observed during phagocytosis of A. fumigatus hyphae. After incubation with hyphae, monolayers were fixed and A. fumigatus stained with 10 μg mL−1 calcofluor white (white). Actin stained with phalloidin (green). Image representative of ≥30 fields from ≥2 separate experiments. Scale bar: 5 μm.