Figure 1.
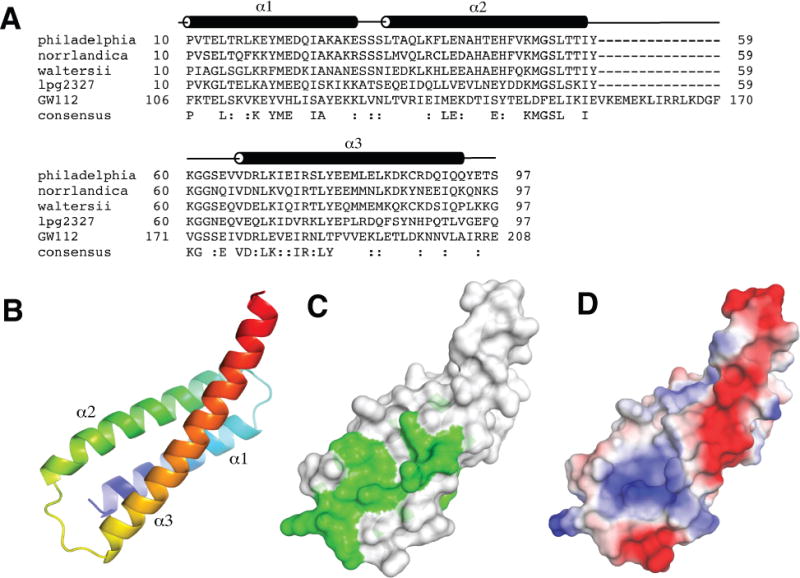
Structure of Lem22. (A) Sequence alignment of Lem22 from Legionella pneumophila (NCBI reference YP_096337), Legionella norrlandica (WP_035889725), Legionella waltersii (WP_058479910), N-terminal domain of lpg2327 from Legionella pneumophila (AEW52564) and a homologous region of human GW112 (XP_014637891). The positions of α-helices (α1-α3) are shown according to the structure from L. pneumophila. (B) Cartoon representation of lpg2328 structure with the molecule colored from blue at the N-terminus to red at the C-terminus. (C) Sequence conservation mapped to the structure. Green color corresponds to highly conserved residues. (D) Surface charge representation of lpg2328. Red color represents negative charge, blue color - positive charge. The surface charge was generated with PyMOL with electrostatic potential values ranging from -67.3 to 67.3.
