Fig. 2.
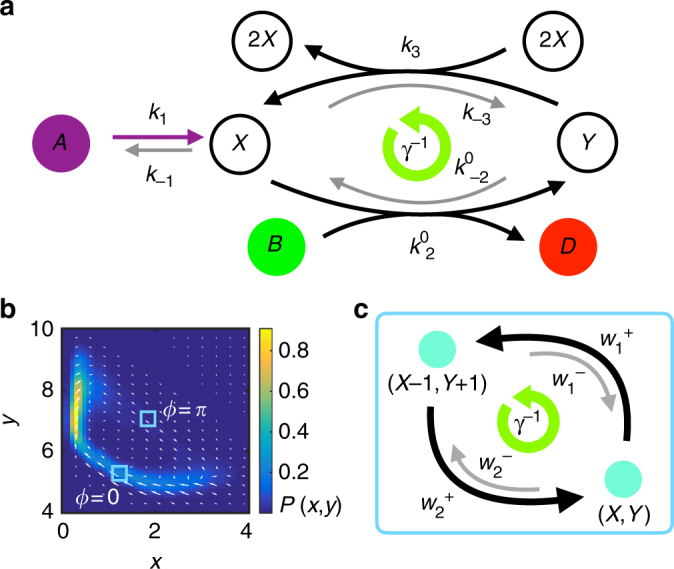
The reversible Brusselator model. a The reaction with B and D can be mapped into a unimolecular reaction with rate constants: , . Together with the autocatalytic reaction with rates k3 and k−3, they form a reaction cycle with a reversibility parameter γ ≡ k−2k−3/k2k3. When γ ≠ 1, the system is nonequilibrium with free-energy dissipation driving the cyclic flux X → Y → X. b The steady-state probability density P(x, y) (color plot) and the state-space fluxes (Jx, Jy) (vector field) of the model. The two small blue boxes highlight the regions around the two opposite phases ϕ = 0 and ϕ = π in the deterministic limit cycle. c Details of the chemical reactions in a local region (e.g., the small boxes in b) in the state space. Two microscopic states, (X − 1, Y + 1) and (X, Y), are linked by two distinct reversible reaction pathways, which form a microscopic reaction cycle (loop)
