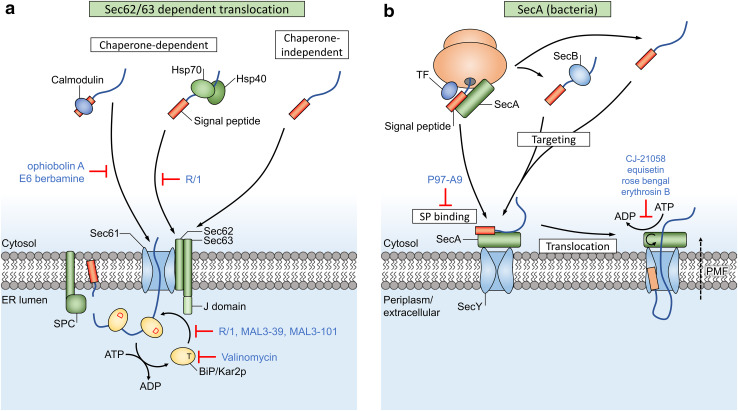
Fig. 4.
Sec-dependent post-translational translocation of pre-proteins involves several chaperone proteins. a Calmodulin binds signal peptides (orange box) and is targeted to Sec61, as Sec61 contains a cytosolic signal peptide-binding site. Hsp chaperones retain cytosolic proteins in a translocation-competent state and are targeted to Sec62/63. Other chaperone-independent targeting pathways are possible too, e.g., with intrinsically disordered proteins. The J domain of Sec63 converts BiP to a state with high affinity for protein binding. Sequential binding of BiP molecules works as a ratcheting mechanism that drives post-translational translocation. b Targeting of bacterial pre-proteins can occur either in a chaperone-dependent way through the help of SecA, SecB or trigger factor (TF), or in a chaperone-independent way. SecY-dependent post-translational translocation in bacteria relies on the essential ATPase motor protein SecA and the proton motive force (PMF) to drive translocation of pre-proteins through the SecY pore and across the plasma membrane. Inhibitors of translocation are indicated in blue text