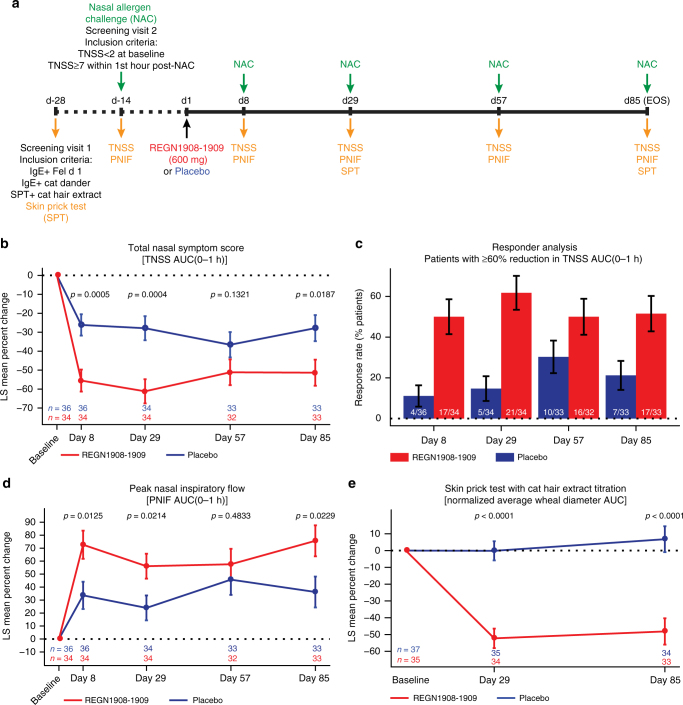
Fig. 5.
REGN1908–1909 blocks the early phase allergic response to cat extract in patients with cat allergy. a Schematic of the study design. b Percent change from baseline in total nasal symptom score (TNSS) AUC(0–1 h); LS mean ± SE is shown for the full analysis set (FAS). c Percentage of patients with ≥60% reduction in TNSS AUC(0–1 h) is shown at each study timepoint. Numbers on the bars indicate the number of patients represented out of the patient number measured at each timepoint. Percentage of patients ± SE is shown for the FAS. d Percent change from baseline in peak nasal inspiratory flow (PNIF) AUC(0–1 h); LS mean ± SE is shown for the FAS. e Percent change from baseline in normalized average wheal diameter AUC is shown from the titrated (100-33,000SQU) cat hair extract skin prick test read 15 min after administration on study days 29 and 85. The skin prick test was not administered on study day 8 or day 57 as per protocol. LS mean ± SE is shown for the safety analysis set. Analyses are based on ANCOVA model with treatment group as a factor and baseline as a covariate. For secondary efficacy and exploratory endpoints no control for multiplicity was performed, therefore p values for all panels are considered nominal