Abstract.
Selenium (Se) is an essential trace element that is involved in numerous biological processes in the form of a selenoprotein such as iodothyronine deiodinase (DIO). Se deficiency may prevent the conversion of T4 to T3 through reducing DIO expression and thereby affecting thyroid hormone status. However, this has not been well documented in humans. In this study, to clarify the association between Se and thyroid hormone status, we investigated the thyroid hormone levels in patients with severe Se deficiency (< 2 µg/dl). Severe Se deficiency was associated with increases in free T4 levels, but not with decreases and increases in free T3 and thyroid stimulating hormone (TSH) levels, respectively. Increases in free T4 levels during Se deficiency were reduced with Se supplementation; however, neither free T3 nor TSH levels were affected. Taken together, these findings indicate that free T4 may be a useful biomarker for Se status when serum Se levels are severely low.
Keywords: selenium, thyroid hormone, deiodinase
Introduction
Selenium (Se) is an essential trace element involved in numerous biological processes including the antioxidant defense system (1, 2), and the biological functions of Se are achieved in the form of selenoproteins. Se deficiency is clinically associated with the development of nail whitening, hair loss, hair color change, muscle weakness, and cardiomyopathy, and Se status is also associated with the development of cancer, cardiovascular disease, diabetes mellitus, and thyroid disease (1, 3, 4); however, the precise molecular mechanisms through which Se status affects the pathogenesis of these diseases are not fully understood (1). The various forms of Se ingested from the diet are converted to selenide. Selenide is then added to tRNA[Ser]Sec in the form of selenophosphate to produce Sec-tRNA[Ser]Sec, and selenocysteine (Sec) is incorporated into selenoproteins during translation with other interacting factors such as SBP2 (selenocysteine insertion sequence-binding protein) (2, 5). Twenty-five selenoproteins, including glutathione peroxidase (GPx) and iodothyronine deiodinases (DIO) (6) have been identified in humans, and decreases in their synthesis have been linked to Se deficiency (7). Se intake differs markedly among countries and is based on the Se content in the environment (8). Se deficiency therefore rarely occurs in countries such as Japan where Se content in the environment is sufficient. However, Se deficiency can be common in certain clinical settings where patients are exclusively nourished through parenteral nutrition or enteral formula that may not include Se supplementation (9).
DIO is one of the selenoproteins involved in thyroid hormone metabolism. DIO1 and DIO2 catalyze the synthesis of the active thyroid hormone T3 from T4 (10). Therefore, Se deficiency may result in increases and decreases in T4 and T3 levels, respectively, through reducing the expression and/or activities of DIO1 and DIO2. This has been demonstrated in a study in which rodents that had been fed a low Se diet showed increased T4 levels with decreased T3 levels (7). Furthermore, mutations in the SBP2 gene, which are critical for the incorporation of Se during translation, have been reported to increase and decrease T4 and T3 levels, respectively, in humans (11). Consistent with these observations, the inverse relationship between Se concentration and T4 levels have been reported (12); however, there is also evidence that demonstrates a lack of association between these two parameters (13). These findings suggest that the effect of Se deficiency on thyroid hormone status remains in dispute, especially in humans. To clarify the association between Se status and thyroid hormone levels in humans, we retrospectively analyzed the thyroid hormone status in patients with Se deficiency and found that Se deficiency was associated with increases in free T4 levels.
Methods
Study design and patients
We retrospectively evaluated the medical records of 15 patients (ranging from 6 mo to 24 yr old) who presented with severe Se deficiency. Severe Se deficiency was defined as Se levels below the detection limit (less than 2.0 μg/dl). All patients had visited the Osaka Women’s and Children’s Hospital prior to December 2015, and their clinical characteristics are summarized in Table 1. Serum levels of TSH (thyroid stimulating hormone), FT3 (free T3), FT4 (free T4), and MCV (mean corpuscular volume) were evaluated based on medical records. Serum TSH, FT3, and FT4 levels were analyzed based on competitive enzyme immunoassay (Tosoh AIA Automated Immunoassay System, Tosoh Bioscience, Inc., Japan), and serum Se levels were determined using an atomic absorption spectrometry-based method. The study was approved by the Ethical Review Board of Osaka Women’s and Children’s Hospital.
Table 1. Clinical characteristics of patients.
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). Results were analyzed for significant differences using the paired Student’s t-test. Pearson’s correlation coefficients were used to analyze associations. Significance was set at p < 0.05.
Results
Clinical characteristics of the patients
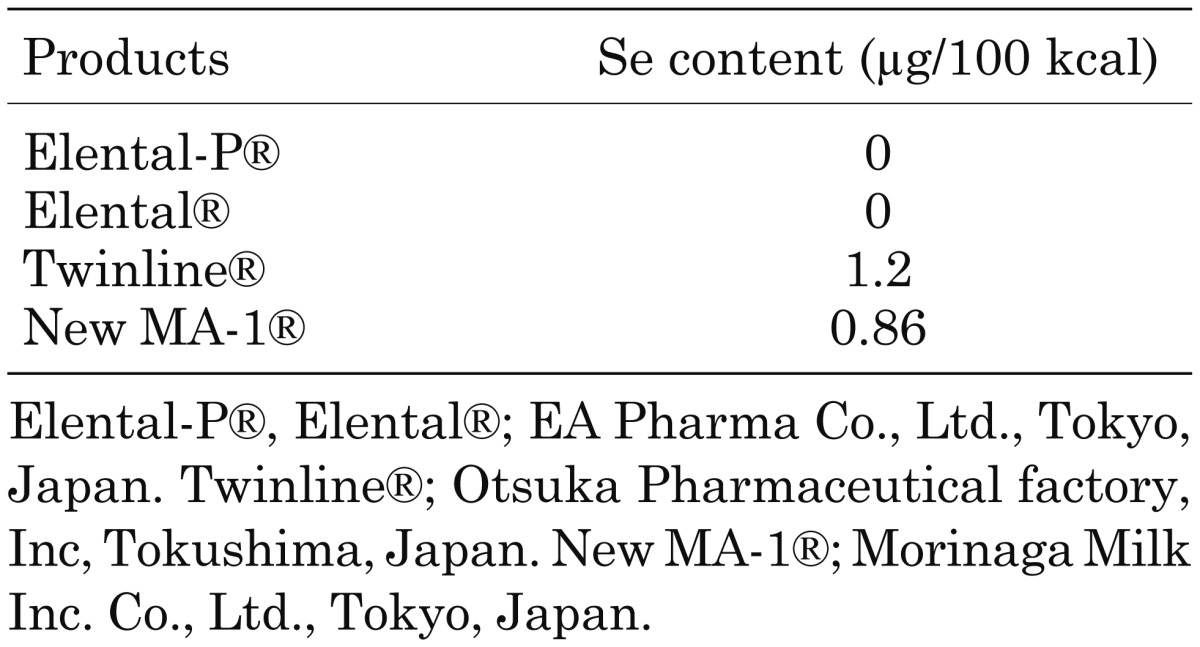
The clinical characteristics of 15 patients with severe Se deficiency are summarized in Table 1. Eleven patients were exclusively nourished through enteral feeding and 4 were nourished through total parenteral nutrition. Se content in the enteral formula is summarized in Table 2. Se supplementation was provided through a normal diet, Se-containing enteral supplements (V-ACCEL®, NUTRI Co., Ltd. Mie, Japan), and/or intravenous Selenic acid (H2SeO4) administration, and the duration of Se supplementation ranged from 3 months to 18 months, as shown in Table 1.
Table 2. Se content in enteral formula.
Free T4 levels were elevated in patients with severe selenium deficiency
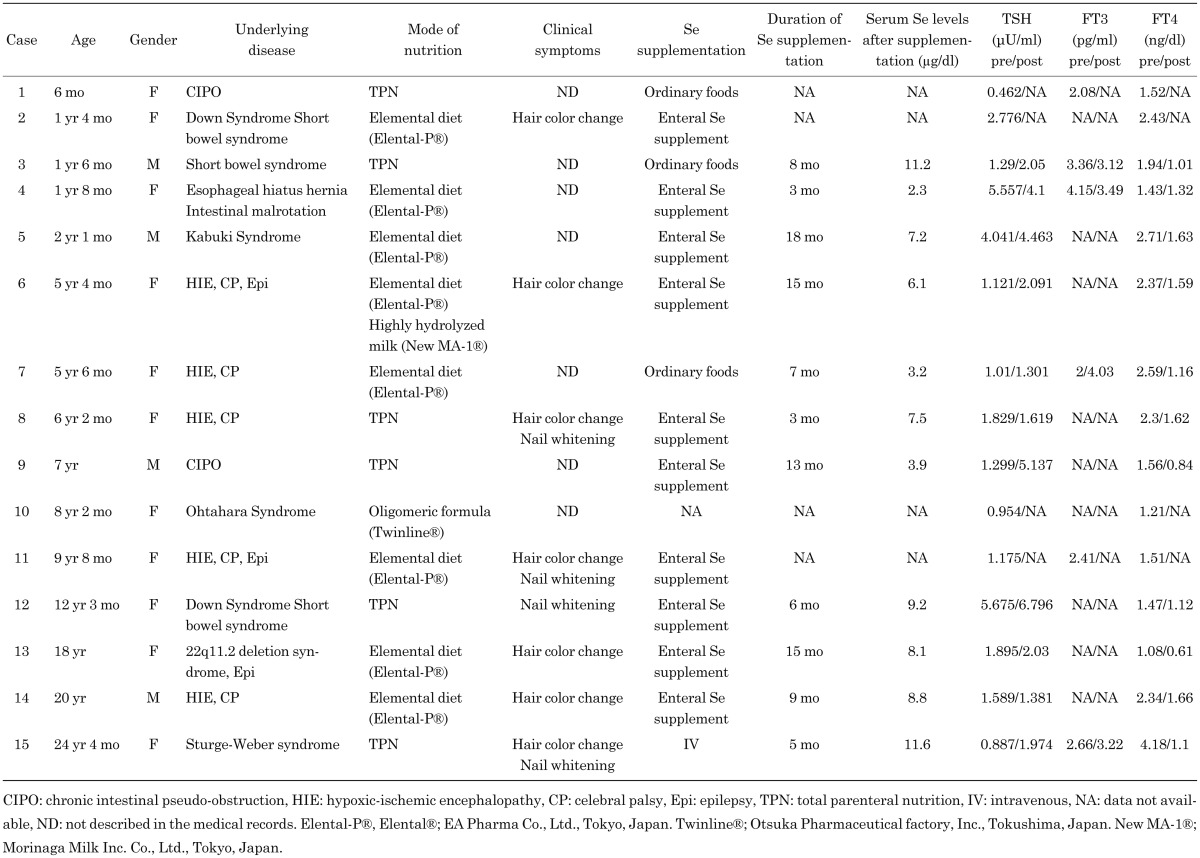
To investigate the association between Se deficiency and thyroid hormone status, the serum concentrations of TSH, FT3, and FT4 levels in the patients were retrospectively determined based on their medical records. The TSH and FT4 levels were measured in all patients, while the FT3 levels were only available in 9 patients. As shown in Fig. 1, the mean FT4 levels were elevated (institutional normal range, 0.82–1.63 ng/dl). Seven of 15 patients showed FT4 levels above the normal range, and 5 of 9 patients showed either low or low-normal levels of FT3 with the average levels of FT3 within the institutional normal range (2.0–4.9 pg/ml). The TSH levels were slightly elevated in 2 patients, but the average levels fell within normal values (institutional normal range, 0.41–4.01 µU/ml). The average levels of MCV, which are known to be elevated in patients with Se deficiency, were within the normal range (89–99 fl), and only 4 of 15 patients showed increases in MCV levels.
Fig. 1.
FT4 levels are elevated in patients with severe Se deficiency. Serum levels of TSH (N = 15), FT3 (N = 9), FT4 (N = 15), and MCV (N = 15) were evaluated in patients who displayed severe Se deficiency. Broken lines indicate the upper and lower limits of the institutional normal range. Closed circles represent the values of each patient. The mean value and standard deviation are shown on the right.
Supplementation of Se was associated with decreases in FT4 levels
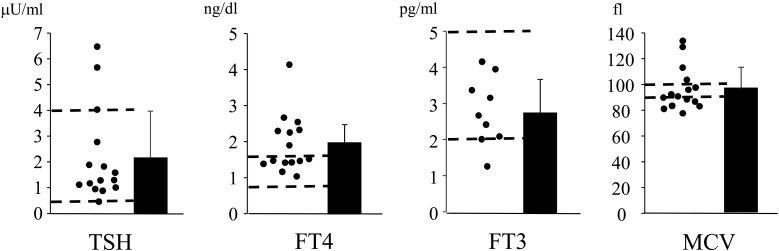
To determine whether elevation of the FT4 levels was due to Se deficiency, changes in thyroid hormone levels were analyzed after Se supplementation. The thyroid hormone levels were compared in the 11 patients receiving Se supplementation (Table 1). Although the serum Se levels were elevated due to Se supplementation, the levels still met the Se deficiency criteria, as set by the Japanese Society of Clinical Nutrition, in 5 patients (0–5 yr, ≤ 6.0 µg/dl; 6–14 yr, ≤ 7.0 µg/dl; 15–18 yr, ≤ 8.0 µg/dl; and >19 yr, ≤ 10.0 µg/dl). The reason the increase in Se levels differed among participants may be due to the distinct mode, amount, and duration of Se supplementation, as intravenous Se supplementation rapidly achieved the normalization of Se concentration compared to the other mode of Se supplementation. In response to the increased Se levels, the FT4 levels decreased significantly, and all the patients showed normal levels of FT4; however, the levels of TSH and FT3 did not alter with Se supplementation (Fig. 2 and Table 1).
Fig. 2.
FT4 levels are decreased after Se supplementation. Serum levels of TSH (N = 11), FT3 (N = 4), FT4 (N = 11), and MCV (N = 11) were compared before and after Se supplementation. Closed circles represent the values of each patient. Values from the same patient are connected in a straight line.
No association was observed between serum Se and FT4 levels in patients after Se supplementation
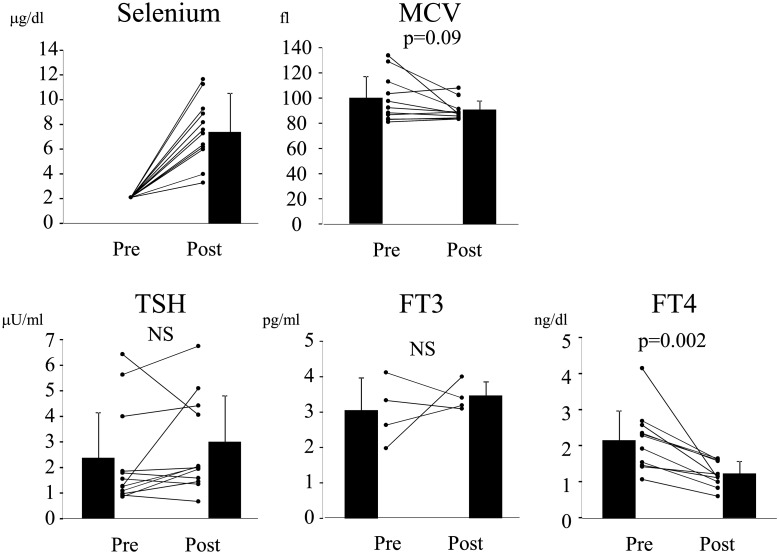
We determined whether Se concentration was associated with FT4 levels in patients who had displayed detectable Se levels after Se supplementation. As shown in Fig. 3A and B, neither FT4 nor MCV correlated with Se levels. In addition, increases in Se levels were not associated with changes in FT4 levels (Fig. 3C). These findings suggest that FT4 does not reflect the state of Se sufficiency when the Se content is mildly decreased.
Fig. 3.
No association is observed between Se and FT4 levels after Se supplementation. (A) Correlation between serum Se and FT4 levels were analyzed. Pearson’s correlation coefficient r = –0.0025, p = 0.99. (B) Correlation between serum Se levels and MCV was determined. Pearson’s correlation coefficient r = 0.24, p = 0.42. (C) Correlation between increases in serum Se levels (ΔSelenium: subtracting 2.0 from the values obtained after Se supplementation) and the changes in FT4 levels (ΔFT4) was determined. Pearson’s correlation coefficient r = –0.36, p = 0.20.
Discussion
In this study, we showed that decreases in Se levels were associated with increased T4 levels in patients with severe Se deficiency. DIO1 and DIO2 are selenoproteins, and they have been shown to be decreased in conditions of Se deficiency. The mechanism through which Se deficiency elevates FT4 levels most likely involves the suppression of DIO1 and DIO2 expressions that catalyze the conversion of T4 to T3. Similar findings have also been reported in elderly people (12), and in patients with phenylketonuria (14) and cystic fibrosis (15). However, a randomized controlled trial involving 368 euthyroid participants who exhibited low-to-moderate Se deficiency demonstrated no association between Se status and the T4/T3 ratio (13). Therefore, the role of Se in thyroid hormone metabolism has demonstrated inconsistency between studies and remains unclear.
As parenteral nutrition or enteral formula may not include Se supplementation, Se deficiency can occur in given clinical settings. Indeed, Se levels have been shown to be decreased in many of the patients nourished through parenteral nutrition (16, 17), but no clinical symptoms have been reported in patients with Se deficiency (16), suggesting that Se deficiency-associated clinical symptoms occur more frequently as Se deficiency extends in duration. However, there is also evidence that Se deficiency-associated symptoms emerged shortly after Se became deficient (9); therefore it is still unclear whether there is an association between the duration of Se deficiency and the development of clinical symptoms.
Animal studies have revealed that Se content differs significantly within tissues during a state of Se deficiency. For example, the Se content in the cerebrum, pituitary, and thyroid is maintained compared to the liver and kidney (7, 18). Consistent with this, the DIO activity is also preserved in the former tissues (18). These findings indicate that physiologically important tissues are protected from Se deficiency; however, the underlying mechanisms that cause the uneven distribution of Se during Se deficiency remain to be elucidated. Since DIO activity is minimally affected when Se intake is moderately reduced (7), T4 levels and/or the T4/T3 ratio are unlikely to be affected in a state of moderate Se deficiency. This hypothesis was supported in the previously mentioned randomized controlled study (13). In contrast, when Se intake is severely reduced, impaired DIO activity seems to cause the elevation of T4 and/or the T4/T3 levels through blocking the T4 conversion to T3. This suggests that differences in the definition of Se deficiency among studies may have led to different conclusions regarding the association between Se and the thyroid hormone status.
Our findings have shown that Se status does not affect TSH levels, which may also indicate that Se deficiency is not associated with hypothyroidism. However, caution should be taken when interpreting the association between thyroid hormone status and TSH levels because the Se content differs among the tissues during Se deficiency. As described above, the Se content is greater in the cerebrum and in the pituitary gland where a negative feedback system is operative, implying that T3 conversion from T4 in these tissues is maintained compared to the other tissues. These findings suggest that the serum concentration of TSH levels may not reflect the peripheral thyroid hormone status, and it is possible that TSH levels are inappropriately low despite the presence of peripheral thyroid hormone deficiency.
Se deficiency causes growth retardation in both rodents and humans; however, the underlying mechanisms are not fully understood. Since Se deficiency has been shown to reduce GPx activity, the accumulation of oxidative damage may explain the mechanisms of Se deficiency-associated growth retardation (19, 20). It is possible that hypothyroidism due to Se deficiency is responsible for Se deficiency-associated growth retardation; however, further studies are required to confirm this hypothesis.
In conclusion, we found that serum FT4 levels were elevated in patients with severe Se deficiency but were reduced with Se supplementation. Therefore, these findings highlight the use of FT4 levels as a possible biomarker for severe Se deficiency. Understanding the alterations in thyroid hormone levels in conditions of Se deficiency is important to allow for early intervention concerning Se deficiency prior to the development of Se deficiency-associated clinical symptoms. Since mutations in the SBP2 gene also cause inhibition in the conversion of T4 to T3, this disorder should be considered as a differential diagnosis when increases in FT4 levels are associated with decreases in FT3 and marginal increases in TSH levels (11, 21,22,23,24). Since the TSH levels did not decrease with Se supplementation, it is still unclear whether Se deficiency can cause the development of hypothyroidism. Further investigations are required to clarify the association between Se deficiency and hypothyroidism.
References
- 1.Rayman MP. Selenium and human health. Lancet 2012;379: 1256–68. doi: 10.1016/S0140-6736(11)61452-9 [DOI] [PubMed] [Google Scholar]
- 2.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973;179: 588–90. doi: 10.1126/science.179.4073.588 [DOI] [PubMed] [Google Scholar]
- 3.Wu Q, Rayman MP, Lv H, Schomburg L, Cui B, Gao C, et al. Low population selenium status is associated with increased prevalence of thyroid disease. J Clin Endocrinol Metab 2015;100: 4037–47. doi: 10.1210/jc.2015-2222 [DOI] [PubMed] [Google Scholar]
- 4.Plogsted S, Adams SC, Allen K, Cober MP, Greaves J, Mogensen KM, et al. Nutrition Product Shortage Subcommittee, Clinical Practice Committee, American Society for Parenteral and Enteral Nutrition. Parenteral nutrition trace element product shortage considerations. Nutr Clin Pract 2016;31: 843–7. doi: 10.1177/0884533616670374 [DOI] [PubMed] [Google Scholar]
- 5.Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev 2014;94: 739–77. doi: 10.1152/physrev.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, et al. Characterization of mammalian selenoproteomes. Science 2003;300: 1439–43. doi: 10.1126/science.1083516 [DOI] [PubMed] [Google Scholar]
- 7.Bermano G, Nicol F, Dyer JA, Sunde RA, Beckett GJ, Arthur JR, et al. Tissue-specific regulation of selenoenzyme gene expression during selenium deficiency in rats. Biochem J 1995;311: 425–30. doi: 10.1042/bj3110425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkel LH, Vriens B, Jones GD, Schneider LS, Pilon-Smits E, Bañuelos GS. Selenium cycling across soil-plant-atmosphere interfaces: a critical review. Nutrients 2015;7: 4199–239. doi: 10.3390/nu7064199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etani Y, Nishimoto Y, Kawamoto K, Yamada H, Shouji Y, Kawahara H, et al. Selenium deficiency in children and adolescents nourished by parenteral nutrition and/or selenium-deficient enteral formula. J Trace Elem Med Biol 2014;28: 409–13. doi: 10.1016/j.jtemb.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 10.Schomburg L. Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol 2011;8: 160–71. doi: 10.1038/nrendo.2011.174 [DOI] [PubMed] [Google Scholar]
- 11.Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 2005;37: 1247–52. doi: 10.1038/ng1654 [DOI] [PubMed] [Google Scholar]
- 12.Olivieri O, Girelli D, Azzini M, Stanzial AM, Russo C, Ferroni M, et al. Low selenium status in the elderly influences thyroid hormones. Clin Sci (Lond) 1995;89: 637–42. doi: 10.1042/cs0890637 [DOI] [PubMed] [Google Scholar]
- 13.Rayman MP, Thompson AJ, Bekaert B, Catterick J, Galassini R, Hall E, et al. Randomized controlled trial of the effect of selenium supplementation on thyroid function in the elderly in the United Kingdom. Am J Clin Nutr 2008;87: 370–8. [DOI] [PubMed] [Google Scholar]
- 14.Calomme MR, Vanderpas JB, François B, Van Caillie-Bertrand M, Herchuelz A, Vanovervelt N, et al. Thyroid function parameters during a selenium repletion/depletion study in phenylketonuric subjects. Experientia 1995;51: 1208–15. doi: 10.1007/BF01944738 [DOI] [PubMed] [Google Scholar]
- 15.Kauf E, Dawczynski H, Jahreis G, Janitzky E, Winnefeld K. Sodium selenite therapy and thyroid-hormone status in cystic fibrosis and congenital hypothyroidism. Biol Trace Elem Res 1994;40: 247–53. doi: 10.1007/BF02950797 [DOI] [PubMed] [Google Scholar]
- 16.Lipkin E, Schumann L, Young JH, Ivey M. Prediction of whole blood selenium levels in patients on long-term parenteral nutrition. JPEN J Parenter Enteral Nutr 1986;10: 40–4. doi: 10.1177/014860718601000140 [DOI] [PubMed] [Google Scholar]
- 17.Fleming CR, McCall JT, OBrien JF, Forsman RW, Ilstrup DM, Petz J. Selenium status in patients receiving home parenteral nutrition. JPEN J Parenter Enteral Nutr 1984;8: 258–62. doi: 10.1177/0148607184008003258 [DOI] [PubMed] [Google Scholar]
- 18.Bates JM, Spate VL, Morris JS, St Germain DL, Galton VA. Effects of selenium deficiency on tissue selenium content, deiodinase activity, and thyroid hormone economy in the rat during development. Endocrinology 2000;141: 2490–500. doi: 10.1210/endo.141.7.7571 [DOI] [PubMed] [Google Scholar]
- 19.Nogales F, Ojeda ML, Fenutría M, Murillo ML, Carreras O. Role of selenium and glutathione peroxidase on development, growth, and oxidative balance in rat offspring. Reproduction 2013;146: 659–67. doi: 10.1530/REP-13-0267 [DOI] [PubMed] [Google Scholar]
- 20.Mistry HD, Broughton Pipkin F, Redman CW, Poston L. Selenium in reproductive health. Am J Obstet Gynecol 2012;206: 21–30. doi: 10.1016/j.ajog.2011.07.034 [DOI] [PubMed] [Google Scholar]
- 21.Hamajima T, Mushimoto Y, Kobayashi H, Saito Y, Onigata K. Novel compound heterozygous mutations in the SBP2 gene: characteristic clinical manifestations and the implications of GH and triiodothyronine in longitudinal bone growth and maturation. Eur J Endocrinol 2012;166: 757–64. doi: 10.1530/EJE-11-0812 [DOI] [PubMed] [Google Scholar]
- 22.Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam O, et al. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest 2010;120: 4220–35. doi: 10.1172/JCI43653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Cosmo C, McLellan N, Liao XH, Khanna KK, Weiss RE, Papp L, et al. Clinical and molecular characterization of a novel selenocysteine insertion sequence-binding protein 2 (SBP2) gene mutation (R128X). J Clin Endocrinol Metab 2009;94: 4003–9. doi: 10.1210/jc.2009-0686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azevedo MF, Barra GB, Naves LA, Ribeiro Velasco LF, Godoy Garcia Castro P, de Castro LC, et al. Selenoprotein-related disease in a young girl caused by nonsense mutations in the SBP2 gene. J Clin Endocrinol Metab 2010;95: 4066–71. doi: 10.1210/jc.2009-2611 [DOI] [PubMed] [Google Scholar]