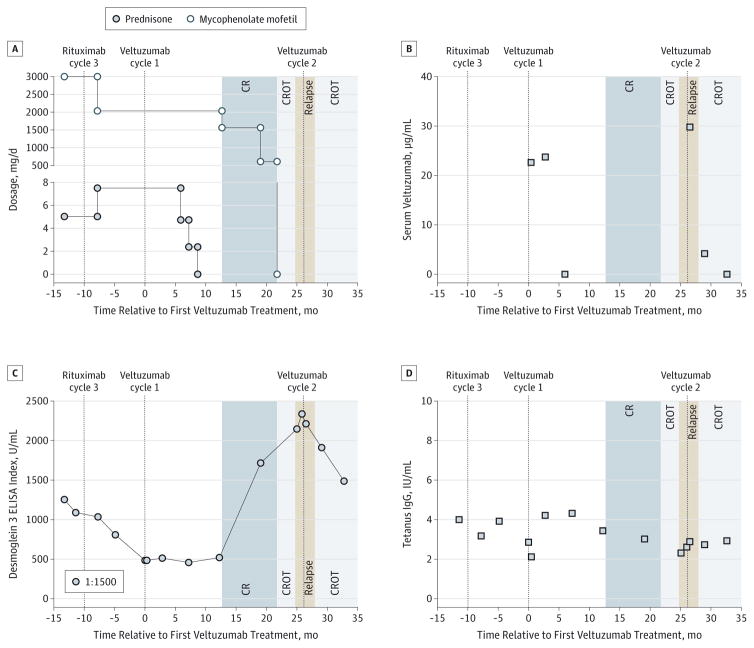
Figure 1. Evaluation of Clinical Course, Treatments, Pharmacokinetics, and Response to Therapy.
A, Immunosuppressant dosages and clinical outcomes. CR, complete remission (minimal therapy); CROT, CR off therapy. B, Pharmacokinetic analysis. Serum veltuzumab levels were 22 and 23 μg/mL and 29 and 4 μg/mL when measured at 0.5 and 3.0 months after veltuzumab cycles 1 and 2, respectively. C, Modified (1:1500) desmoglein 3 enzyme-linked immunosorbent assay (ELISA), which correlates with relapse and remission before and after veltuzumab cycle 2. D, Serum tetanus toxoid IgG remained stable.