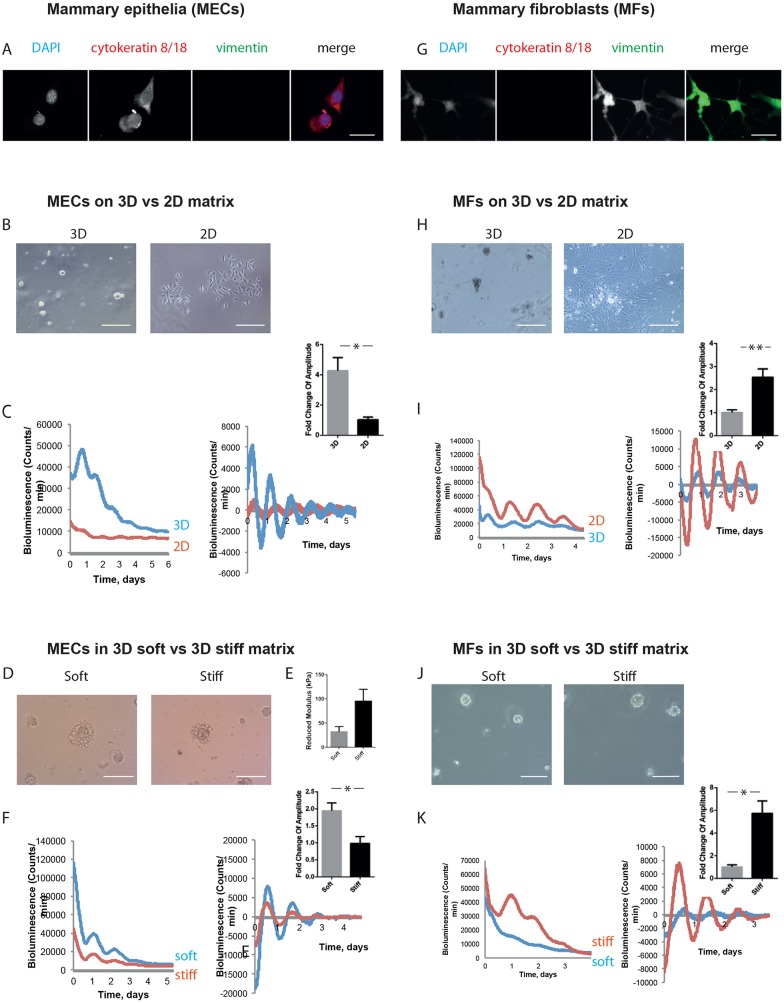
Fig. 1.
Inverse mechano-sensitivity of mammary epithelial and fibroblast circadian clocks. (A) Mammary epithelial cells (MECs) were isolated, and a pure epithelial population was confirmed by cytokeratin-positive and vimentin-negative staining. DAPI, blue; cytokeratin 8/18, red; vimentin, green (single-channel images shown in grey scale). Scale bar: 50 µm. (B) Phase-contrast images of MECs cultured in 3D and 2D. Scale bars: 50 µm. (C) MECs show larger amplitude of oscillation when cultured on 3D than 2D. Bioluminescence traces presented as raw (left) and normalised (right), with fold-change graph above. (Student's t-test, P<0.05, mean±s.e.m.). (D) Phase-contrast images of MECs cultured in soft and stiff alginate gels. Scale bar: 50µm. (E) Stiffness of soft and stiff gels, revealed by atomic force microscopy. (Student's t-test, P<0.05, mean±s.e.m.). (F) MECs show larger amplitude of oscillation when cultured in soft alginate gels rather than stiff gels. Bioluminescence traces presented as raw (left) and normalised (right), with fold-change graph above. (Student's t-test, P<0.05, mean±s.e.m.). (G) Mammary fibroblasts (MFs) were isolated by FACS. Immunofluorescence staining revealed MFs are cytokeratin-negative and vimentin-positive. DAPI, blue; cytokeratin 8/18, red; vimentin, green (single-channel images shown in grey scale). Scale bar: 50 µm. (H) Phase-contrast images of MFs cultured in 3D and 2D. Scale bars: 50 µm. (I) MFs show larger amplitude of oscillation when cultured on 2D plastic than 3D. Bioluminescence traces presented as raw (left) and normalised (right), with fold-change graph above. (Student's t-test, P<0.05, mean±s.e.m.). (J) Phase-contrast images of MFs cultured in soft and stiff alginate gels. Scale bar: 50 µm. (K) MFs show larger amplitude of oscillation when cultured in stiff alginate gels rather than soft gels. Bioluminescence traces presented as raw (left) and normalised (right), with fold-change graph above. (Student's t-test, P<0.05, mean±s.e.m.). For MECs, 3 independent biological replicates were performed, each with 2 animals, which were pooled together to form 2 cultures per condition. For MFs, 3 independent biological replicates were performed, each with 3 animals, which were pooled together to form 2 cultures per condition.