Fig. 1.
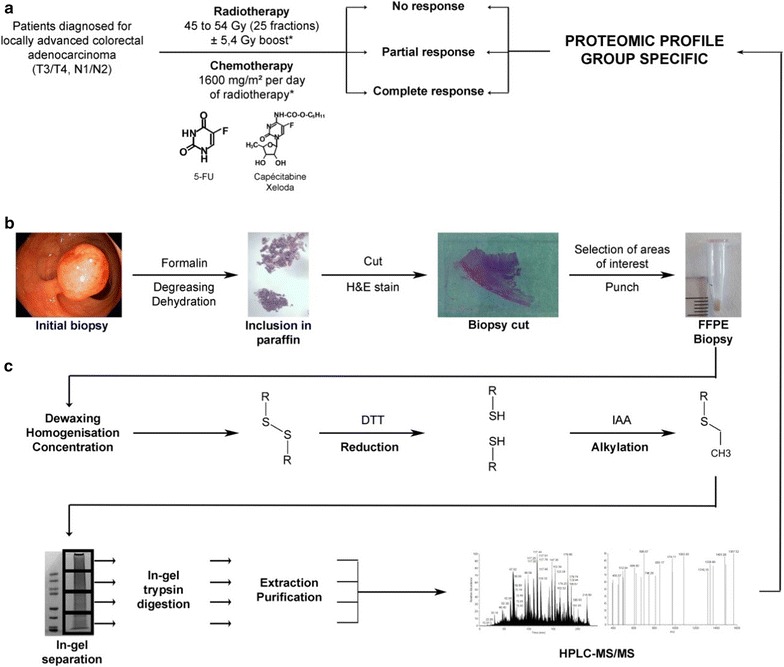
Overview of the workflow to obtain the group-specific proteomic profile. a Clinical selection and treatment. Patients with locally advanced non-metastatic colorectal cancer (CRC) are treated by radiotherapy (RT) with a concomitant chemotherapy (CT), in a neoadjuvant situation (preoperatively) (NRCT). (*) The doses indicated are the standard doses applied. The dose of RT is 45 to 54 Gray (Gy) in total, divided into 25 fractions (5 days per week for 5 weeks). The concomitant CT is 1600 mg/m2 per day of RT. It can be either intravenous (5-fluorouracil, 5-FU) or oral (Capecitabine or Xeloda®) (in the majority of cases, unless contraindications). After treatment, patients are divided in three groups according to their response to the treatment: no response (NR; absence of decreased stage/size of the tumor), partial response (PR; decreased stage/size of the tumor), and complete response (TR; elimination of the tumor). b From the initial sample to the FFPE biopsy: the physiopathological step. The fixation in formalin is immediately realized after the biopsy. The degreasing and dehydration are necessary for the inclusion in paraffin. The cutting and H&E staining steps allowed the pathologist to select the areas of interest and make a punch to obtain the FFPE biopsy (more details in Fig. 2). Punches are realized with TMA Master II (3DHISTECH). All these steps are realized in the laboratory of pathology. c From the FFPE biopsy to its proteomic profile: the experimental step. First steps (dewaxing, homogenisation and concentration) are necessary to realize a dithiothréitol reduction and an iodoacetamide alkylation on the samples. These last are in-gel separated and trypsin digested, and then the peptides are extracted and purified before their separation on HPLC–MS/MS. Analysis are realized with different software such as MaxQuant software package version 1.5.2.8 [18] and Perseus software package [19] and allowed to establish a proteomic profile specific for each group of responders. 5-FU 5-fluorouracil, CRC colorectal cancer, CT chemotherapy, FFPE formalin-fixed paraffin-embedded, Gy Gray, H&E haematoxylin and eosin, HPLC–MS/MS high performance liquid chromatography separation coupled to mass spectrometry, NR non-responders, PR partial responders, RT radiotherapy, TMA tissue microarray, TR total responders
