Fig. 3.
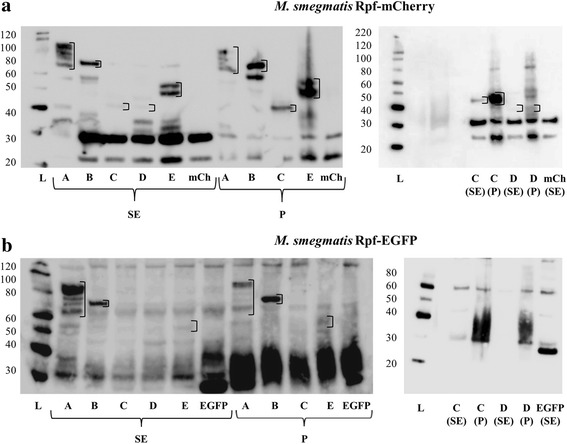
Localisation of Rpf-mCherry/EGFP fusions by western blot: protein extracts. The samples were extracted as detailed in Methods. SDS-PAGE gels were loaded with approximately 20 μg of soluble extract (SE; total proteins) and 5 μl of precipitated extract (P), previously resuspended in 50 μl of loading buffer. The letters A-E correspond to samples from RpfA-E-mCherry/EGFP-producing cultures, respectively. mCh and EGFP refer to the control strains harbouring control plasmids. The molecular weight ladder (L) values in kDa are shown on the left of each gel. Empty squares have been included encircling the bands of interest. a The left western blot performed with Rpfs-mCherry producing strains presents bands at the expected heights for all the fusion proteins in the SE and P. In some cases (RpfA and RpfE) more than one band appears per sample, most probably due to protein degradation/aggregation. The right blot contains repeat RfpC-mCherry samples and RpfD-mCherry pellet (that is not present on the left). The results show that these two proteins are present in both SE and P, but are more concentrated in the P fraction. b In the left blot performed with Rpfs-EGFP producing strains, RpfC-EGFP could not be identified in the SE or P. The right blot contains repeat RfpC-mCherry samples (as this protein could not be identified on the left) and RpfD-mCherry pellet (that was not present in the WB on the left). These two proteins could not be identified in the SE or P. Hypothetical molecular weight of the fusion proteins: RpfA-EFFP/mCherry (≈67 kDa), RpfB-EGFP/mCherry (≈65 kDa), RpfCEGFP/mCherry (≈45 kDa), RpfD-EGFP/mCherry (≈43 kDa), RpfE-EGFP/mCherry (≈46 kDa)
