Abstract
Background:
The olfactory groove meningioma has always been surgically challenging. The common microscopic surgical procedures exercised involve modification of pterional or sub-frontal approaches with or without orbital osteotomies. However, we believe that orbital osteotomies are not mandatory to achieve gross total resection. Hence, this study was performed to evaluate the surgical outcomes of olfactory groove meningioma with bicoronal sub frontal approach but without orbital osteotomies.
Materials and Methods:
The study was performed by reviewing the medical charts, neuroimaging data, and follow-up data of 19 patients who were treated micro surgically for olfactory groove meningioma without orbital osteotomies in our department. Mean overall follow up period of our study was 5 years. Statistical analysis was done by means of IBM SPSS Software version 19.
Results:
Nineteen patients (1 male and 18 female patients, with an age range of 35-67 years; average age of patients' 51±7.5 years) of OGM were managed in our department. All patients were evaluated by MRI Brain with and without Gadolinium, CTA, CT Scan both axial and Coronal sequences. Most common symptom reported was head ache (80%), others include; urinary incontinence (26%), seizures (78%), decreased visual acuity (79%), papilledema (74%), personality changes (68%) and olfactory loss was reported in 57% of the patients. Post-operative complications include; CSF accumulation (5%), hematoma at tumor bed (10%), skin infection (5%) and mild post-operative brain edema (26%). Mortality rate was 5%. During 5 years of follow-up, we recorded one recurrence which was after 26 months and successfully removed in reoperation.
Conclusion:
Bi-coronal sub frontal approach appears to be an excellent technique for Olfactory Meningioma removal as practiced by most neurosurgeons. Nevertheless, it is not mandatory to carry out orbital osteotomy to acquire optimal surgical outcome as is advocated by some Authors.
Keywords: Bicorporal subfrontal approach, cerebrospinal fluid, olfactory groove meningioma, tuberculum sellae meningioma
Introduction
Olfactory groove meningiomas (OGMs) take their origin from the cribriform plate and frontoethmoidal suture in the floor of anterior cranial base.[1,2] These meningiomas are thought to arise from arachnoid cap cells of arachnoid granulations in this region[3] and comprise 10% of all meningiomas in the brain.[4] Endo-skull base approaches are gaining popularity due to the advent of sophisticated endoscopic instruments and specialized training in this area of modern neurosurgery with comparable morbidity and mortality.[5,6,7,8,9] Open approaches are still gold standard worldwide including our institution for OGM. These approaches are a modification of pterional or subfrontal approaches with or without orbital osteotomies.[4,10,11,12]
Due to large size of the tumors in our series (6 cm ± 2 mean), we utilized bicoronal subfrontal craniotomy without orbital osteotomy due to following advantages:
Reaching its main bleeders' anterior and posterior ethmoidal arteries early in the course of the resection of the tumors, especially staying extradurally early in the operation
Safe separation of the tumors from the optic nerve, chiasm, anterior cerebral artery, and frontal lobe with minimal retraction
Visualization and reconstruction of anterior cranial base and repair of its defects after resection, especially ethmoidal air sinuses if involved.[4,10,11]
We therefore preferred bicoronal subfrontal approach to attempt gross-total resection in all cases and believe addition of orbital osteotomy is not mandatory to achieve gross-total resection as opposed to most authors who used orbital osteotomies.[4,13,14] These authors believe that less brain retraction, wide working corridor, and better control of intraorbital ethmoidal arteries are achieved through orbital osteotomies to achieve Grade 1 resection. Orbital osteotomies increase bony work, distort natural anatomy, and increase operative time and blood loss. We achieved the same goals without concomitant disadvantages of orbital osteotomies and present our result.
Materials and Methods
After the study was approved by Research Ethics Committee of our institution, data of 19 patients were collected using medical records, postoperative surgeon's notes, and follow-up visits. Preoperative evaluation was performed by means of magnetic resonance imaging (MRI) brain with and without contrast. T2-weighted image and fluid-attenuated inversion recovery (FLAIR) sequences were utilized to know any surrounding brain edema or pial invasion which is important in surgical planning. Computed tomography (CT) scan of both sagittal and coronal reconstruction was done to look for anterior skull base invasion, ethmoid sinuses involvement, and hyperostotic bone. Angiography with intent of embolization was not done as ethmoidal arteries are branches of ophthalmic arteries and embolization is risky in this region. However, we obtained CT angiogram (CTA) to know the relation of the tumor with vasculature. Especial emphasis was placed to look for any residual that was left behind by doing MRI with contrast within 2 days and immediates postoperative CT scan to look for brain swelling and postoperative hematoma. Every preoperative and postoperative neurological deficit was examined by senior residents. The follow-up period of this study was 5 years.
Imaging features
OGM behaves similar to other meningioma on CT scan. They were hyperdense on CT scan compared to normal brain and characteristically enhanced homogeneously. On T1 MRI, they appeared isointense to gray matter, and on T2-weighted sequences, they were variable; hyperintense to gray matter when it was soft tumor and hypointense when more fibrous and calcified element were noted during surgery. They brightly enhanced on contrast administration. The relation of surrounding vasculature that supplies the tumor was nicely delineated by CTA. Catheter angiogram with the option of embolization was not carried out for any case in our experience.
Treatment options
OGM often presents when they are large enough where conservative policy does not work except in the case of elderly patients with many comorbidities. Thus, we offered surgery as the first option to all patients. Other options such as observation and radiosurgery were not used in our series.
Surgical technique
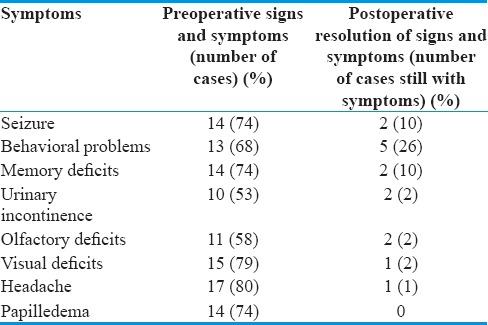
After the general anesthesia was administered, we placed lumbar drain in all our patients with lumbar puncture needle and closed it. One gram third generation cephalosporin antibiotic, 10 mg dexamethasone, and 1 g of phenytoin were administered intravenously to all patients preoperatively. Two hundred milliliter of mannitol was then administered after the skin flap was turned. Patients having preoperative seizure were maintained on antiepileptic for 1 year while those who did not have seizure were placed on antiepileptic for 1 week postoperatively. Relatively more hydration was maintained during surgery. Lumbar drain was closed until the opening of dura and drained around 20 ml of cerebrospinal fluid (CSF) to get maximum brain relaxation, especially before reaching the cistern. We operated all patients in the supine position by bifrontal craniotomy starting with skin incision from zygoma to zygoma (Soutar type) after Mayfield three pins fixation with slight head extension to get the use of gravity so as the frontal lobe fall back. Incision was placed within 1 cm of tragus to avoid injury to the superficial temporal artery. Pericranium was raised as a separate layer along with its pedicle of blood vessels to use it in the reconstruction of the anterior skull base. Two burr holes were placed at keyholes bilaterally on frontosphenoid suture 6-mm posterior to frontozygomatic suture. Other two burr holes one on each side of the sagittal sinus in the midline were also placed to straddle the sinus. Sinus dura was released from the undersurface of the bone using blunt Penfield dissector. Craniotome was used to complete the craniotomy to incorporate the frontal sinus flushed with anterior cranial base in each case. While crossing the sinus, we do not use foot flat of craniotome. The frontal sinuses were cranialized by removing its mucosa and posterior wall. Crista galli was removed. We did not perform orbital osteotomies in our cases. Meticulous dural coagulation was performed and dural vessels were coagulated. At this stage, microscope was brought into the field. We elevated dura from the anterior cranial base and devascularized the tumor staying extradurally. We coagulated the anterior and posterior ethmoidal arteries at this stage. After this maneuver, dura was opened in a straight line along the frontal poles. Sagittal sinus was sutured and cut along with falx just above the crista galli. No complications were seen due to sacrifice of the sinus in this region in our experience. Due to the effect of gravity, frontal lobes were easily retracted and tumor was easily approached. Fixed retractor on the frontal lobes was avoided at all the time. This was facilitated by lumbar drain and draining CSF from the nearby cisterns. Suction tip was enough for the purpose of retraction. Meticulous extracapsular coagulation was performed and internal debulking was done initially. Capsule was then moved toward center and good plane was developed between capsule and the surrounding brain. Because meningiomas are dural based and push arachnoid ahead of them; thus, they are situated in the subdural space outside arachnoid. This pathoanatomic configuration was observed in all our cases, and we found this configuration of great help during dissection of the tumor respecting that arachnoid layer to avoid injuries to vessels.[15] We had difficulty visualizing the optic chiasm and anterior communicating artery complex initially as can be expected, and with patience and tumor removal from anterior to posterior, we ultimately were able to visualize the optic apparatus and anterior cerebral arteries and its branches nicely. We separated the tumor capsule nicely from this important neurovascular structure without any injury except one tumor which was adherent to vessels. We left a small residual of the tumor behind in this patient. Tumors invading into the optic canal were easily removed just by sectioning of falciform ligament and decompressing the optic nerve. This was possible by an excellent trajectory from the anterior aspect to the medial optic canal bilaterally where most of the tumor was found. In our experience, the medial compartment of the optic canal was filled with tumor and pushing the optic nerve laterally against the anterior clinoid process. We felt it to be unnecessary to perform clinoidectomy just thinking heat generated by drill may be damaging to the optic nerve. Although many surgeons have performed it successfully. Two tumors invaded the upper half of the ethmoid sinuses, and we removed this part by entering the ethmoid sinuses from above just angling the microscope into the ethmoid sinus, and at this point, slight more CSF was released from the lumbar drain with very minimal retraction applied to the frontal lobe and took that part without any difficulty. This approach is an excellent option to deal with ethmoidal sinus, and many modifications can be made at this juncture if the tumor cannot be delivered without frontal lobe retraction. In our experience, we did not modify our approach and were able to reach the ethmoidal sinus just tilting microscope rather than modifying the approach by extra bony work. We did not encounter any retraction-related injury in these two cases. We were able to achieve Grade 1 Simpson resection in all cases except one in which we achieved Grade 2 Simpson due to tumor attachment to neurovascular structures [Table 1].
Table 1.
Preoperative symptomatology compared to postoperative resolution in our series
Reconstruction of anterior cranial base
Vascularized pericranium was raised as separate layer initially during the procedure and reflected inferiorly in wet gauze along with its blood pedicle. After the removal of the tumor and drilling the hyperostotic bone, this vascularized pericranium was laid over the base of the anterior cranial base, and two stitches were applied on each side to the base of anterior fossa to secure it in place. Frontal lobe provided buttress effect on the vascularized flap. In case of the ethmoidal sinuses opening, after removing the mucosa and tumor, sinus was packed with mashed bone from the burr hole and frontal sinus bone then fat graft was placed over it. Fibrin glue was applied to hold it in place and finally pericranium was laid over the entire anterior cranial base. We did not experience any case of rhinorrhea in all our patients. Dura was closed in standard fashion. Galea and skin closed as usual and subgaleal drain was not placed.
Results
In the present study, ratio of male to female was 1:18 and the average age at presentation was 51 ± 7.5 years and range was 35–67 years are comparable to other studies [Table 2].
Table 2.
The clinical features of the recent series with ours
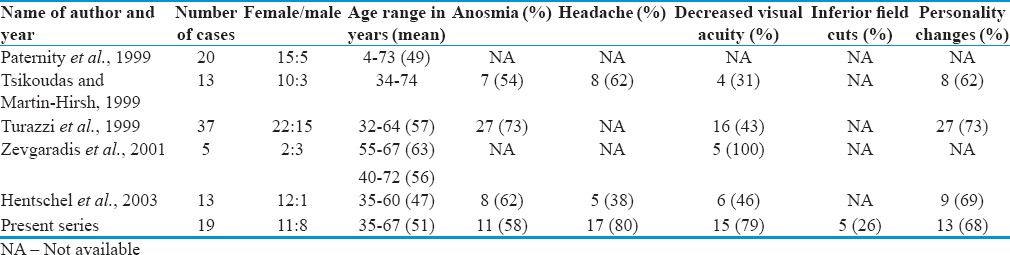
In our series, urinary incontinence and seizure were seen in 5 of 19 (26%) and 14 of 19 (78%) patients, respectively, that are not reported in the above five series. Headache was the most common symptom reported by 17 of 19 (80%) patients.
Of the visual symptoms, decreased visual acuity was most common and seen in 15 of 19 (79%) patients while inferior field cuts were seen in 5 of 19 (26%) patients. Papilledema was seen 14 of 19 patients (74%). These were significantly improved after resection of the tumor and cutting of falciparum ligament with the removal of the tumor from the optic canal. Personality changes were seen in 13 of 19 (68%) patients. Elderly patients were mostly affected by confusion along with memory loss. Olfactory loss was reported by 11 of 19 (57%) patients bilaterally. Anatomically, olfactory tracts were thinned out by the tumor.
We observed excellent clinical improvement in the preoperative symptoms after the resection of the tumor. Fourteen of 19 patients (78%) presented with seizure preoperatively, and only 2 patients had seizure postoperatively that responded to single antiepileptic drug for 1 year and successfully weaned off after year of therapy. Two new patients developed seizure after surgery, and they also responded well to single epileptic for 1 year. Memory and behavior problems improved significantly, and only 2 of 14 (14%) patients were having memory deficits for 6 months that improved slowly overtime. Five of 19 (26%) patients presented with urinary incontinence preoperatively and only 2 patients are still having urinary incontinence postoperatively [Table 1]. There was 81% (9 of 11 patients) of improvement in olfaction and 2 still with olfactory loss. We found thinned out olfactory nerve in these patients. Anatomic integrity of the olfactory tract was maintained during dissection of the tumor from the olfactory tract in these cases.
Of 15 patients having decreased visual acuity, one patient still has decreased visual acuity although optic nerves were decompressed in this patient bilaterally by cutting falciform ligaments and removing the tumor from the medial optic canal. Papilledema resolved in all 14 cases.
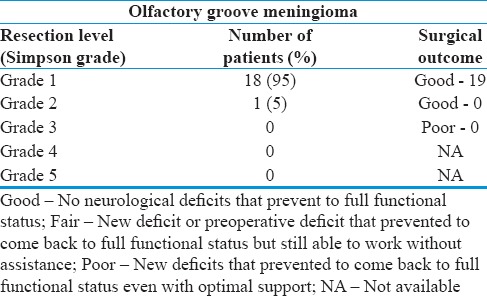
With this approach, we achieved Grade 1 Sampson resection in all patients except one case in which neurovascular structure could not be separated from the tumor where Grade 2 resection was achieved. Tumor from the ethmoid sinuses was removed successfully just by tilting the microscope to get straight trajectory into the sinus easily; no extra-frontal lobe retraction was applied. We believe that lumbar drain is extremely useful to give brain relaxation in such cases, especially if we could not get into the nearby cistern to aspirate CSF early in the course of dissection. We were able to remove the tumor piecemeal from the sinus with ease, and no CSF leak was observed in these two patients postoperatively [Table 3].
Table 3.
Resection level in our series
We observed one case of transient CSF leak from incision and swelling of the incision site due to underlying CSF accumulation in pseudomeningocele fashion. CT scan was done to see if there is underlying hydrocephalus. CT scan did not show any hydrocephalus and that swelling disappeared after 3 days just with slight pressure bandage. CSF stopped spontaneously. Two of 19 (10%) patients developed postoperative hematoma at tumor bed. This resolved spontaneously in repeat CT scan. Two patients developed new seizure after the resection of the tumor and were placed on phenytoin for 1 year. The seizure was well controlled on the phenytoin that was tapered after 1 year successfully. One of 19 (5%) patients had skin site infection that required antibiotic for 2 weeks and resolved. Five of 19 (26%) patients developed mild postoperative brain edema that required mannitol and dexamethasone for 5 days and resolved without any complication. T2-weighted MRI and FLAIR sequence in these patients did not show pial invasion or surrounding brain edema preoperatively. We did not know why it happened even though we did not use extra brain retraction or fix retraction in these cases [Table 4].
Table 4.
Postsurgical complications and outcome
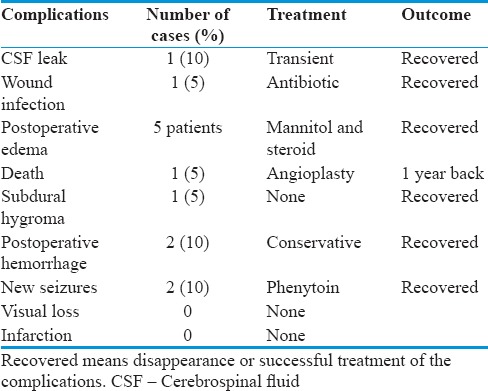
One of 19 (5%) patients had postoperative hygroma in subdural space that resolved without any complication.
Of all the patients, 1 patient (5%) died 2 days after the operation, he had angioplasty 1 year back and was declared fit for this surgery preoperatively. No any infarction or worsening visual deficits postoperatively were noted in our series of 19 patients.
Discussion
OGM derives their main blood supply from anterior and posterior ethmoidal arteries which are branches of the ophthalmic artery. Some blood supply is also derived from the branches of anterior meningeal arteries, anterior communicating, and anterior cerebral arteries defending on their posterior superior extension.[16,17] Headaches and visual symptoms are common symptoms that are progressive as the tumor size enlarges. As these tumors compress optic apparatus from above and push them downward, the finding on visual field assessment is usually located inferiorly. Five of 19 (26%) patients had inferior cuts that responded very well to section of falciform ligament and removal of tumor from the medial optic nerve canal without performing anterior clinoidectomy. Subfrontal approach provided excellent view from anterior perspective after removing the tumor. This panoramic view greatly facilitated sectioning of falciform ligament and decompression of both optic nerves, not crossing the nerves rather working between the nerves. Crossing optic nerves makes them more vulnerable to the injury during dissection. Anosmia is reported by a large number of patients and can be picked up by careful clinical examination. Foster Kennedy syndrome that is unilateral optic atrophy and contralateral papilledema was first described in OGM. This syndrome is rarely seen in OGM. In our series of patients, we only observed papilledema in 14 of 19 (74%) patients that resolved with resection of the tumor.
One of the most important differential diagnoses in this region is tuberculum sellae meningiomas (TSMs). OGM pushes optic apparatus downward and laterally as the tumor grows from anterior side while TSM pushes it upward and laterally as this tumor grows from posterior aspect.[4,17,18] Therefore, optic apparatus is more likely damaged during the resection of TSM as compared to OGM. The goal of surgery is total resection when possible and recurrence depends on the level of resection. In the present study, we preferred bicoronal subfrontal approach in all cases due to its advantages mentioned earlier and relatively large size of the tumors. There was an excellent improvement in the preoperative symptomatology after removal of the tumors [Table 1].
Patients having personality disorders, visual and memory deficits improved significantly. Turazzi et al.[11] and few others[19] reported similar results. These patients had long-standing symptoms and were attributed to neuropsychiatric disorders. Olfaction in our patients was interestingly regained in 9 of 11 patients and two were left with permanent loss although anatomic preservation of olfactory tract was seen in all our cases. However, we did not assess olfaction by special tests rather we used clinical examination and asking the patients if they could recognize particular odors.
Welge-Luessen et al.[20] reported that none of their two patients had olfaction even after preserving the olfactory nerve anatomically on that side.
As they grow, they push arachnoid and make folding of it around itself to ultimately form two layers of arachnoid. The outer layer of arachnoid contains neurovascular structure; thus, tumor dissection was performed between the tumor capsule and outer arachnoid though in larger tumors, this appeared as single layer. Preservation of these arachnoid layers facilitated the safer microsurgical dissection of the tumors from the neurovascular structure in our experience [Table 3]. We were able to achieve Sampson Grade 1 resection in 18 of 19 (95%) patients and Grade 2 in 1 patient using standard microsurgical techniques. Others[21,22] also reported a similar level of resection. Even with this level of resection, morbidity has been very low using microsurgical techniques in the recent series compared to ones in the past.[5,11,21,23,24]
Complications of the surgical management of OGM include CSF leak, meningitis, postoperative hemorrhage, subdural hygroma, visual disturbance, postoperative brain swelling, postoperative seizures, and new neurologic deficits.[25,26] The complication rate in our series of patients is slightly low compared to other recent series [Table 4].[23,25]
CSF leak has been of great concern during total resection requiring the removal of cribriform flat and dura of anterior cranial base. This has been reported in the range of 0%–24%. Obeid and Al-Mefty[23] reported 20% leak of CSF in their patients (3 of 15 patients) in a report of 80 patients. We used vascularized pericranial flap as carpet to cover anterior cranial base defect and frontal sinus. We found this very useful. We only observed one case of CSF from pressure of the pseudomeningocele like collection of CSF under the incision sit and caused slight swelling which resolved with slight pressure bandage. In two of our patients, tumor was invading the superior part of the ethmoid sinuses that was removed just by tilting the microscope and some more drainage from the lumbar drain to get more relaxation of the brain. This method provided great room to go into the superior part of ethmoid sinuses from above and removed the tumor piecemeal. We enlarged preexisting hole made by invasion of tumor slightly more by drilling the superior bony wall of the ethmoidal sinuses to remove the tumor completely. We packed ethmoid sinuses with mashed bone obtained from burr holes and frontal sinus bone then covered by fat graft and finally glued it with fibrin. We used this method also for the frontoethmoidal encephalocele repair. We found this technique very successful in our hands. The vascularized flap was then placed to cover the anterior cranial base and ethmoid sinuses. We did not encounter rhinorrhea in these two patients. This has been used by several other authors.[15,27]
Mortality rate ranges from 0% to 33% in earlier series,[11,17,20,28,29,30,31] and with the introduction of modern microsurgical techniques and new generation well-illuminated microscopes, mortality in the recent series including our series has significantly decreased.[5,11,17,32,33] One patient (5%) in our series died. This patient had angioplasty 1 year back and declared fit for the surgery. We were unable to determine the cause of his death.
One (5%) patient came back with recurrence after 26 months and again operated through the same bifrontal approach, and Grade 1 resection was possible. Other authors reported recurrence in the range of 0%–41% with mean follow-up of 3.7–25 years.[15,27,29,34]
Conclusion
Bicoronal subfrontal approach appears to be still gold standard for large OGM removal as practiced by most neurosurgeons due to less brain retraction early devascularization of the tumor. Nevertheless, it is not mandatory to carry out orbital osteotomy to acquire optimal surgical outcome as is advocated by some authors. Foremost advantages of excluding orbital osteotomy are that distortion of natural anatomy is prevented along with increased operating time and blood loss meanwhile achieving the desired surgical outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.DeMonte F, Marmor E, Al-Mefty O. In: Brain Tumor: An Encyclopedic Approach. 2nd ed. Kaye A, Laws E Jr, editors. London: Churchill Livingstone; 2001. pp. 719–50. [Google Scholar]
- 2.Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: Analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18–24. doi: 10.3171/jns.1985.62.1.0018. [DOI] [PubMed] [Google Scholar]
- 3.Kepes JJ. Meningioma: Biology, Pathology and Differential Diagnosis. New York: Masson; 1982. [Google Scholar]
- 4.Hentschel SJ, Demonte F. Olfactory groove meningioma. Neurosurg Focus. 2003;14:1–5. doi: 10.3171/foc.2003.14.6.4. [DOI] [PubMed] [Google Scholar]
- 5.de Divitiis E, Esposito F, Cappabianca P, Cavallo LM, de Divitiis O, Esposito I. Endoscopic transnasal resection of anterior cranial fossa meningiomas. Neurosurg Focus. 2008;25:E8. doi: 10.3171/FOC.2008.25.12.E8. [DOI] [PubMed] [Google Scholar]
- 6.Dehdashti AR, Ganna A, Witterick I, Gentili F. Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: Indications and limitations. Neurosurgery. 2009;64:677–87. doi: 10.1227/01.NEU.0000339121.20101.85. [DOI] [PubMed] [Google Scholar]
- 7.Gardner PA, Kassam AB, Thomas A, Snyderman CH, Carrau RL, Mintz AH, et al. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery. 2008;63:36–52. doi: 10.1227/01.NEU.0000335069.30319.1E. [DOI] [PubMed] [Google Scholar]
- 8.Greenfield JP, Anand VK, Kacker A, Seibert MJ, Singh A, Brown SM, et al. Endoscopic endonasal transethmoidal transcribriform transfovea ethmoidalis approach to the anterior cranial fossa and skull base. Neurosurgery. 2010;66:883–92. doi: 10.1227/01.neu.0000368395.82329.c4. [DOI] [PubMed] [Google Scholar]
- 9.Kassam AB, Thomas A, Carrau RL, Snyderman CH, Vescan A, Prevedello D, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008;63(1) Suppl 1:ONS44–52. doi: 10.1227/01.neu.0000297074.13423.f5. [DOI] [PubMed] [Google Scholar]
- 10.Paterniti S, Fiore P, Levita A, La Camera A, Cambria S. Venous saving in olfactory meningioma's surgery. Clin Neurol Neurosurg. 1999;101:235–7. doi: 10.1016/s0303-8467(99)00054-2. [DOI] [PubMed] [Google Scholar]
- 11.Turazzi S, Cristofori L, Gambin R, Bricolo A. The pterional approach for the microsurgical removal of olfactory groove meningiomas. Neurosurgery. 1999;45:821–5. doi: 10.1097/00006123-199910000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Hassler W, Zentner J. Surgical treatment of olfactory groove meningiomas using the pterional approach. Acta Neurochir Suppl (Wien) 1991;53:14–8. [PubMed] [Google Scholar]
- 13.Tsikoudas A, Martin-Hirsch DP. Olfactory groove meningiomas. Clin Otolaryngol. 1999;24:507–9. doi: 10.1046/j.1365-2273.1999.00303.x. [DOI] [PubMed] [Google Scholar]
- 14.Sekhar LN, Nanda A, Sen CN, Snyderman CN, Janecka IP. The extended frontal approach to tumors of the anterior, middle, and posterior skull base. J Neurosurg. 1992;76:198–206. doi: 10.3171/jns.1992.76.2.0198. [DOI] [PubMed] [Google Scholar]
- 15.Alexiou GA, Gogou P, Markoula S, Kyritsis AP. Management of meningiomas. Clin Neurol Neurosurg. 2010;112:177–82. doi: 10.1016/j.clineuro.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Mayfrank L, Gilsbach JM. Interhemispheric approach for microsurgical removal of olfactory groove meningiomas. Br J Neurosurg. 1996;10:541–5. doi: 10.1080/02688699646835. [DOI] [PubMed] [Google Scholar]
- 17.Ojemann RG. Olfactory groove meningiomas. In: Al-Mefty O, editor. Meningioma. New York: Raven Press; 1991. pp. 383–93. [Google Scholar]
- 18.Jallo GI, Benjamin V. Tuberculum sellae meningiomas: Microsurgical anatomy and surgical technique. Neurosurgery. 2002;51:1432–39. [PubMed] [Google Scholar]
- 19.Zevgaridis D, Medele RJ, Müller A, Hischa AC, Steiger HJ. Meningiomas of the sellar region presenting with visual impairment: Impact of various prognostic factors on surgical outcome in 62 patients. Acta Neurochir (Wien) 2001;143:471–6. doi: 10.1007/s007010170076. [DOI] [PubMed] [Google Scholar]
- 20.Welge-Luessen A, Temmel A, Quint C, Moll B, Wolf S, Hummel T. Olfactory function in patients with olfactory groove meningioma. J Neurol Neurosurg Psychiatry. 2001;70:218–21. doi: 10.1136/jnnp.70.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura M, Struck M, Roser F, Vorkapic P, Samii M. Olfactory groove meningiomas: Clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2008;62(6) Suppl 3:1224–32. doi: 10.1227/01.neu.0000333788.83349.1e. [DOI] [PubMed] [Google Scholar]
- 22.Sami M, Ammirati M. Olfactory groove meningioma. In: Sami M, editor. Surgery of the Skull Base: Meningiomas. Berlin: Springer Verlag; 1992. pp. 15–25. [Google Scholar]
- 23.Obeid F, Al-Mefty O. Recurrence of olfactory groove meningiomas. Neurosurgery. 2003;53:534–42. doi: 10.1227/01.neu.0000079484.19821.4a. [DOI] [PubMed] [Google Scholar]
- 24.Symon L. Olfactory groove and suprasellar meningiomas. In: Krayenbuhl H, editor. Advances and Technical Standards in Neurosurgery. Vienna: Springer Verlag; 1977. pp. 67–91. [Google Scholar]
- 25.El Gindi S. Olfactory groove meningioma: Surgical techniques and pitfalls. Surg Neurol. 2000;54:415–7. doi: 10.1016/s0090-3019(00)00346-3. [DOI] [PubMed] [Google Scholar]
- 26.Solero CL, Giombini S, Morello G. Suprasellar and olfactory meningiomas. Report on a series of 153 personal cases. Acta Neurochir (Wien) 1983;67:181–94. doi: 10.1007/BF01401420. [DOI] [PubMed] [Google Scholar]
- 27.Delfini R, Iannetti G, Belli E, Santoro A, Ciappetta P, Cantore G. Cranio-facial approaches for tumours involving the anterior half of the skull base. Acta Neurochir (Wien) 1993;124:53–60. doi: 10.1007/BF01401122. [DOI] [PubMed] [Google Scholar]
- 28.Bakay L, Cares HL. Olfactory meningiomas. Report on a series of twenty-five cases. Acta Neurochir (Wien) 1972;26:1–12. doi: 10.1007/BF01413528. [DOI] [PubMed] [Google Scholar]
- 29.Chan RC, Thompson GB. Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. A retrospective study in 257 cases. J Neurosurg. 1984;60:52–60. doi: 10.3171/jns.1984.60.1.0052. [DOI] [PubMed] [Google Scholar]
- 30.Holub K. Intrakranielle meningeome. Acta Neurochir (Wien) 1956;4:355–401. doi: 10.1007/BF02106024. [DOI] [PubMed] [Google Scholar]
- 31.Ciric I, Rosenblatt S. Suprasellar meningiomas. Neurosurgery. 2001;49:1372–7. doi: 10.1097/00006123-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Aguiar PH, Tahara A, Almeida AN, Simm R, Silva AN, Maldaun MV, et al. Olfactory groove meningiomas: Approaches and complications. J Clin Neurosci. 2009;16:1168–73. doi: 10.1016/j.jocn.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Aguiar PH, Almeida N. Surgery of olfactory groove meningiomas. In: Ramina R, Henrique P, Aguiar P, Tatagiba M, editors. Sami's Essentials in Neurosurgery. Springer; 2008. pp. 69–77. [Google Scholar]
- 34.DeMonte F. Surgical treatment of anterior basal meningiomas. J Neurooncol. 1996;29:239–48. doi: 10.1007/BF00165654. [DOI] [PubMed] [Google Scholar]


