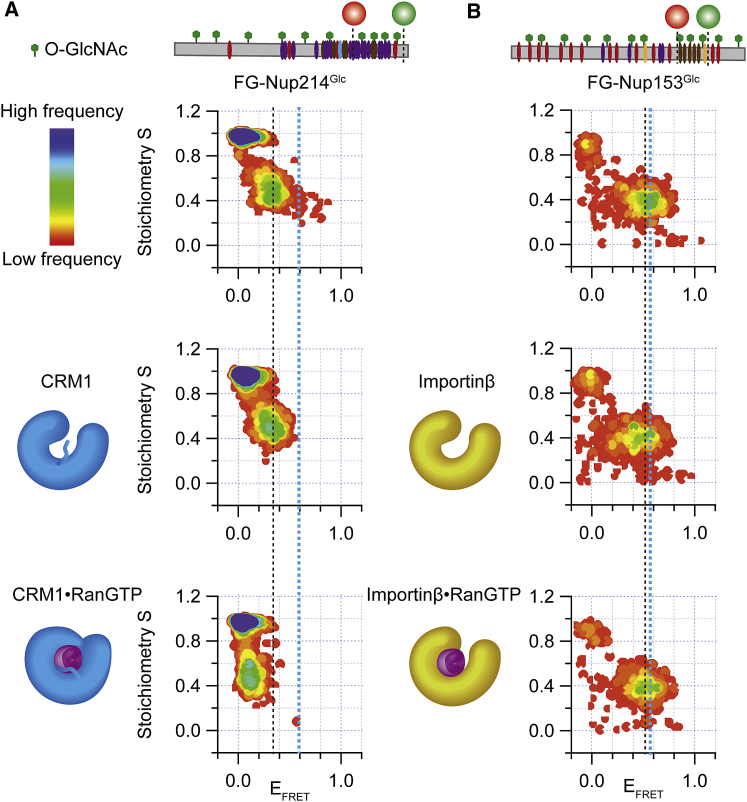
Figure 3.
Conformational Features of Glycosylated FG-Nup upon Interaction with NTR
(A) EFRET versus S histograms of FG-Nup214Glc in the absence and presence of CRM1 and CRM1⋅RanGTP (from top to bottom, at 1 μM for both CRM1 and RanGTP concentrations). In the presence of CRM1, the EFRET population had a similar EFRET value to unbound FG-Nup. In presence of RanGTP, one distinct population with very low EFRET was seen. The EFRET value was remarkably similar to the bound unglycosylated case (compare to Figures 1 and 5).
(B) S versus EFRET histograms of FG-Nup153Glc in the absence and presence of Importinβ and Importinβ⋅RanGTP (from top to bottom, at 1 μM for both Importinβ and RanGTP concentrations). In particular for FG-Nup214Glc, a lower EFRET compared to the unglycosylated state (black dotted line versus blue dotted line) in the unbound form was detected, indicating that the glycosylated Nup is more extended. However, glycosylated and unglycosylated FG-Nups behave remarkably similarly in their respective binding modes, indicating that glycosylation only mildly tunes the binding mechanism between FG-Nups and NTRs. See also Figures S1 and S4.